The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation
- PMID: 28358907
- PMCID: PMC5373613
- DOI: 10.1371/journal.pone.0174754
The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation
Abstract
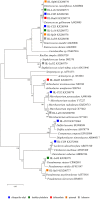
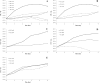
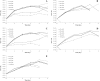
The exploration of new niches for microorganisms capable of degrading recalcitrant molecules is still required. We hypothesized the gut microbiota associated with insect-resistant lines carry pesticide degrading bacteria, and predicted they carry bacteria selected to degrade pesticides they were resistant to. We isolated and accessed the pesticide-degrading capacity of gut bacteria from the gut of fifth instars of Spodoptera frugiperda strains resistant to lambda-cyhalothrin, deltamethrin, chlorpyrifos ethyl, spinosad and lufenuron, using insecticide-selective media. Sixteen isolates belonging to 10 phylotypes were obtained, from which four were also associated with the susceptible strain. However, growth of gut bacteria associated with larvae from the susceptible strain was not obtained in any of the insecticide-based selective media tested. Growth of isolates was affected by the concentration of insecticides in the media, and all grew well up to 40 μg/ml. The insecticide-degrading capacity of selected isolates was assessed by GC or LC-MS/MS analyses. In conclusion, resistant strains of S. frugiperda are an excellent reservoir of insecticide-degrading bacteria with bioremediation potential. Moreover, gut-associated bacteria are subjected to the selection pressure imposed by insecticides on their hosts and may influence the metabolization of pesticides in insects.
Conflict of interest statement
Figures

References
-
- Harris HL, Brennan LJ, Keddie BA, Braig HR. Bacterial symbionts in insects: Balancing life and death. Symbiosis. 2010; 51:37–53.
-
- Feldhaar H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecological Entomology. 2011; 36:533–543.
-
- Douglas AE. The microbial dimension in insect nutritional ecology. Functional Ecology. 2009; 23:38–47.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

