It takes a village to raise a branch: Cellular mechanisms of the initiation of axon collateral branches
- PMID: 28359843
- PMCID: PMC5617777
- DOI: 10.1016/j.mcn.2017.03.007
It takes a village to raise a branch: Cellular mechanisms of the initiation of axon collateral branches
Abstract
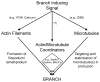
The formation of axon collateral branches from the pre-existing shafts of axons is an important aspect of neurodevelopment and the response of the nervous system to injury. This article provides an overview of the role of the cytoskeleton and signaling mechanisms in the formation of axon collateral branches. Both the actin filament and microtubule components of the cytoskeleton are required for the formation of axon branches. Recent work has begun to shed light on how these two elements of the cytoskeleton are integrated by proteins that functionally or physically link the cytoskeleton. While a number of signaling pathways have been determined as having a role in the formation of axon branches, the complexity of the downstream mechanisms and links to specific signaling pathways remain to be fully determined. The regulation of intra-axonal protein synthesis and organelle function are also emerging as components of signal-induced axon branching. Although much has been learned in the last couple of decades about the mechanistic basis of axon branching we can look forward to continue elucidating this complex biological phenomenon with the aim of understanding how multiple signaling pathways, cytoskeletal regulators and organelles are coordinated locally along the axon to give rise to a branch.
Keywords: Axon sprouting; Filopodia; Interstitial branch; Neurite; Neuronal morphogenesis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interest: The authors have no conflicts of interest to disclose.
Figures



References
-
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

