The activity of cGMP-dependent protein kinase Iα is not directly regulated by oxidation-induced disulfide formation at cysteine 43
- PMID: 28360102
- PMCID: PMC5437233
- DOI: 10.1074/jbc.C117.787358
The activity of cGMP-dependent protein kinase Iα is not directly regulated by oxidation-induced disulfide formation at cysteine 43
Abstract
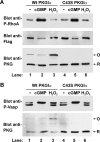
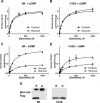
The type I cGMP-dependent protein kinases (PKGs) are key regulators of smooth muscle tone, cardiac hypertrophy, and other physiological processes. The two isoforms PKGIα and PKGIβ are thought to have unique functions because of their tissue-specific expression, different cGMP affinities, and isoform-specific protein-protein interactions. Recently, a non-canonical pathway of PKGIα activation has been proposed, in which PKGIα is activated in a cGMP-independent fashion via oxidation of Cys43, resulting in disulfide formation within the PKGIα N-terminal dimerization domain. A "redox-dead" knock-in mouse containing a C43S mutation exhibits phenotypes consistent with decreased PKGIα signaling, but the detailed mechanism of oxidation-induced PKGIα activation is unknown. Therefore, we examined oxidation-induced activation of PKGIα, and in contrast to previous findings, we observed that disulfide formation at Cys43 does not directly activate PKGIα in vitro or in intact cells. In transfected cells, phosphorylation of Ras homolog gene family member A (RhoA) and vasodilator-stimulated phosphoprotein was increased in response to 8-CPT-cGMP treatment, but not when disulfide formation in PKGIα was induced by H2O2 Using purified enzymes, we found that the Cys43 oxidation had no effect on basal kinase activity or Km and Vmax values; however, PKGIα containing the C43S mutation was less responsive to cGMP-induced activation. This reduction in cGMP affinity may in part explain the PKGIα loss-of-function phenotype of the C43S knock-in mouse. In conclusion, disulfide formation at Cys43 does not directly activate PKGIα, and the C43S-mutant PKGIα has a higher Ka for cGMP. Our results highlight that mutant enzymes should be carefully biochemically characterized before making in vivo inferences.
Keywords: Rho (Rho GTPase); VASP; allosteric regulation; cGMP-dependent protein kinase; cyclic GMP (cGMP); enzyme kinetics; oxidation-reduction (redox).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Ruth P. (1999) Cyclic GMP-dependent protein kinases: understanding in vivo functions by gene targeting. Pharmacol. Ther 82, 355–372 - PubMed
-
- Hoffmann L. S., and Chen H. H. (2014) cGMP: transition from bench to bedside: a report of the 6th International Conference on cGMP Generators, Effectors and Therapeutic Implications. Naunyn Schmiedebergs Arch. Pharmacol. 387, 707–718 - PubMed
-
- Hofmann F., Bernhard D., Lukowski R., and Weinmeister P. (2009) cGMP regulated protein kinases (cGK). Handb. Exp. Pharmacol. 137–162 - PubMed
-
- Schlossmann J., Ammendola A., Ashman K., Zong X., Huber A., Neubauer G., Wang G. X., Allescher H. D., Korth M., Wilm M., Hofmann F., and Ruth P. (2000) Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Iβ. Nature 404, 197–201 - PubMed
-
- Surks H. K., Mochizuki N., Kasai Y., Georgescu S. P., Tang K. M., Ito M., Lincoln T. M., and Mendelsohn M. E. (1999) Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Iα. Science 286, 1583–1587 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

