CD163 Expression in Neurons After Experimental Intracerebral Hemorrhage
- PMID: 28360115
- PMCID: PMC5404936
- DOI: 10.1161/STROKEAHA.117.016850
CD163 Expression in Neurons After Experimental Intracerebral Hemorrhage
Abstract
Background and purpose: CD163, a receptor for hemoglobin, is involved in hemoglobin clearance after intracerebral hemorrhage (ICH). In contrast to microglial/macrophage CD163, neuronal CD163 hemoglobin has not been well studied. This study examined the expression of neuronal CD163 in a pig model of ICH and in vitro rat cortical neurons and the impact of deferoxamine on that expression.
Methods: There were 2 parts to this study. In the in vivo part, piglets had injection of autologous blood into the right frontal lobe. The time course of CD163 expression and the effect of deferoxamine on the expression of CD163 after ICH were determined in the grey matter. In the in vitro part, the levels of CD163 and neuronal death and the effect of deferoxamine were examined in rat cortical neurons culture treated with hemoglobin.
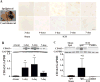
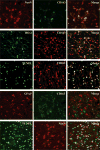
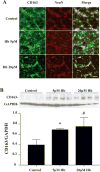
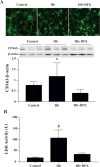
Results: CD163-positive cells were found, and the CD163 protein levels were upregulated in the ipsilateral grey matter after ICH. The CD163 levels peaked at days 1 and 3. The CD163-positive cells were colocated with NeuN-positive, heme oxygenase-2-positive, and terminal deoxynucleatidyl transferase dUTP nick end labeling-positive cells. Deferoxamine treatment attenuated ICH-induced CD163 upregulation and significantly reduced both brain CD163 and hemoglobin levels at day 3. Treating neuronal cultures with hemoglobin for 24 hours resulted in CD163 upregulation and increased cell death. Deferoxamine significantly attenuated the hemoglobin-induced neuronal death and CD163 upregulation.
Conclusions: CD163 is expressed in neurons and upregulated after ICH. Deferoxamine reduced ICH-induced CD163 upregulation and brain cell death in vivo and hemoglobin-induced CD163 upregulation and neuronal death in vitro.
Keywords: cell death; cerebral hemorrhage; deferoxamine; macrophages; swine.
© 2017 American Heart Association, Inc.
Conflict of interest statement
Figures

References
-
- Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. - PubMed
-
- Madsen M, Graversen JH, Moestrup SK. Haptoglobin and cd163: Captor and receptor gating hemoglobin to macrophage lysosomes. Redox Rep. 2001;6:386–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

