Muscle-specific downregulation of GR levels inhibits adipogenesis in porcine intramuscular adipocyte tissue
- PMID: 28360421
- PMCID: PMC5428816
- DOI: 10.1038/s41598-017-00615-9
Muscle-specific downregulation of GR levels inhibits adipogenesis in porcine intramuscular adipocyte tissue
Abstract
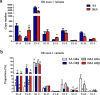
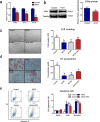
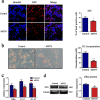
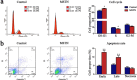
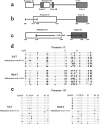
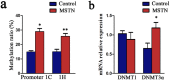
Intramuscular adipose is conducive to good pork quality, whereas subcutaneous adipose is considered as waste in pig production. So uncovering the regulation differences between these two adiposes is helpful to tissue-specific control of fat deposition. In this study, we found the sensitivity to glucocorticoids (GCs) was lower in intramuscular adipocytes (IMA) compared with subcutaneous adipocytes (SA). Comparison of glucocorticoid receptor (GR) revealed that IMA had lower GR level which contributed to its reduced GCs sensitivity. Higher methylation levels of GR promotor 1-C and 1-H were detected in IMA compared with SA. GR expression decrease was also found in adipocytes when treated with muscle conditioned medium (MCM) in vitro, which resulted in significant inhibition of adipocytes proliferation and differentiation. Since abundant myostatin (MSTN) was detected in MCM by ELISA assay, we further investigated the effect of this myokine on adipocytes. MSTN treatment suppressed adipocytes GR expression, cell proliferation and differentiation, which mimicked the effects of MCM. The methylation levels of GR promotor 1-C and 1-H were also elevated after MSTN treatment. Our study reveals the role of GR in muscle fiber inhibition on intramuscular adipocytes, and identifies myostatin as a muscle-derived modulator for adipose GR level.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Hovenier R, Kanis E, Asseldonk TV, Westerink NG. Genetic parameters of pig meat quality traits in a halothane negative population. Livestock Production Science. 1992;32:309–321. doi: 10.1016/0301-6226(92)90002-L. - DOI
-
- Wang S, et al. Glucose utilization, lipid metabolism and BMP-Smad signaling pathway of porcine intramuscular preadipocytes compared with subcutaneous preadipocytes. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31:981–996. doi: 10.1159/000350116. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

