Immunometabolism in early and late stages of rheumatoid arthritis
- PMID: 28360422
- PMCID: PMC6820517
- DOI: 10.1038/nrrheum.2017.49
Immunometabolism in early and late stages of rheumatoid arthritis
Abstract
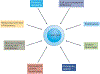
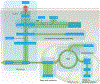
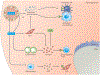
One of the fundamental traits of immune cells in rheumatoid arthritis (RA) is their ability to proliferate, a property shared with the joint-resident cells that form the synovial pannus. The building of biomass imposes high demands for energy and biosynthetic precursors, implicating metabolic control as a basic disease mechanism. During preclinical RA, when autoreactive T cells expand and immunological tolerance is broken, the main sites of disease are the secondary lymphoid tissues. Naive CD4+ T cells from patients with RA have a distinct metabolic signature, characterized by dampened glycolysis, low ATP levels and enhanced shunting of glucose into the pentose phosphate pathway. Equipped with high levels of NADPH and depleted of intracellular reactive oxygen species, such T cells hyperproliferate and acquire proinflammatory effector functions. During clinical RA, immune cells coexist with stromal cells in the acidic milieu of the inflamed joint. This microenvironment is rich in metabolic intermediates that are released into the extracellular space to shape cell-cell communication and the functional activity of tissue-resident cells. Increasing awareness of how metabolites regulate signalling pathways, guide post-translational modifications and condition the tissue microenvironment will help to connect environmental factors with the pathogenic behaviour of T cells in RA.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures

References
-
- Rantapaa-Dahlqvist S et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48, 2741–2749 (2003). - PubMed
-
- Majka DS & Holers VM Can we accurately predict the development of rheumatoid arthritis in the preclinical phase? Arthritis Rheum. 48, 2701–2705 (2003). - PubMed
-
- Arbuckle MR et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 349, 1526–1533 (2003). - PubMed
-
- Kimpimaki T & Knip M Disease-associated autoantibodies as predictive markers of type 1 diabetes mellitus in siblings of affected children. J Pediatr Endocrinol Metab. 14 Suppl 1, 575–587 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

