LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis
- PMID: 28360514
- PMCID: PMC5364009
- DOI: 10.2147/COPD.S130482
LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis
Abstract
Background: Randomized controlled trials (RCTs) indicate that long-acting bronchodilator combinations, such as β2-agonist (LABA)/muscarinic antagonist (LAMA), have favorable efficacy compared with commonly used COPD treatments. The objective of this analysis was to compare the efficacy and safety of LABA/LAMA with LAMA or LABA/inhaled corticosteroid (ICS) in adults with stable moderate-to-very-severe COPD.
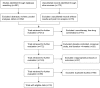
Methods: This systematic review and meta-analysis (PubMed/MEDLINE, Embase, Cochrane Library and clinical trial/manufacturer databases) included RCTs comparing ≥12 weeks' LABA/LAMA treatment with LAMA and/or LABA/ICS (approved doses only). Eligible studies were independently selected by two authors using predefined data fields; the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed.
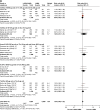
Results: Eighteen studies (23 trials) were eligible (N=20,185). LABA/LAMA significantly improved trough forced expiratory volume in 1 second (FEV1) from baseline to week 12 versus both LAMA and LABA/ICS (0.07 L and 0.08 L, P<0.0001), with patients more likely to achieve clinically important improvements in FEV1 of >100 mL (risk ratio [RR]: 1.33, 95% confidence interval [CI]: [1.20, 1.46] and RR: 1.44, 95% CI: [1.33, 1.56], respectively, the number needed to treat being eight and six, respectively). LABA/LAMA improved transitional dyspnea index and St George's Respiratory Questionnaire scores at week 12 versus LAMA (both P<0.0001), but not versus LABA/ICS, and reduced rescue medication use versus both (P<0.0001 and P=0.001, respectively). LABA/LAMA significantly reduced moderate/severe exacerbation rate compared with LABA/ICS (RR 0.82, 95% CI: [0.75, 0.91]). Adverse event (AE) incidence was no different for LABA/LAMA versus LAMA treatment, but it was lower versus LABA/ICS (RR 0.94, 95% CI: [0.89, 0.99]), including a lower pneumonia risk (RR 0.59, 95% CI: [0.43, 0.81]). LABA/LAMA presented a lower risk for withdrawals due to lack of efficacy versus LAMA (RR: 0.66, 95% CI: [0.51, 0.87]) and due to AEs versus LABA/ICS (RR: 0.83, 95% CI: [0.69, 0.99]).
Conclusion: The greater efficacy and comparable safety profiles observed with LABA/LAMA combinations versus LAMA or LABA/ICS support their potential role as first-line treatment options in COPD. These findings are of direct relevance to clinical practice because we included all currently available LABA/LAMAs and comparators, only at doses approved for clinical use.
Keywords: COPD; LABA/ICS; LABA/LAMA combinations; LAMA; meta-analysis.
Conflict of interest statement
Disclosure GJR has participated as a lecturer, speaker and advisor in scientific meetings and courses under the sponsorship of Air Products and Chemicals Inc, Almirall, AstraZeneca, Boehringer Ingelheim, Laboratorios Dr Esteve, GlaxoSmithKline, Merck Sharp & Dome and Novartis. DP has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Aerocrine, AKL Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals, Theravance, UK National Health Service, Zentiva; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma and Novartis; payment for travel/accommodation/meeting expenses from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; funding for patient enrolment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, and Zentiva; stock/stock options from AKL Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd, UK and 74% of Observational and Pragmatic Research Institute Pte Ltd, Singapore; and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, HTA, and Medical Research Council. AA has acted as a Consultant and has served on advisory boards for Novartis Pharma AG, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Sunnovion. DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies, including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Glenmark, Merck, Napp, Novartis, Pfizer, Respivert, Skyepharma, Takeda, Teva, Therevance and Verona. PA, GB, FP, RF and KK are employees and shareholders of Novartis Pharma AG. KK had previously received honoraria for educational activities and lectures from AstraZeneca, Boehringer Ingelheim, Chiesi, Elpen, and Novartis, and participated on advisory boards arranged by AstraZeneca, Chiesi, Elpen, and Novartis. The authors report no other conflicts of interest in this work.
Figures





References
-
- GOLD [homepage on the Internet] From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. [Accessed December 7, 2016]. Available from: http://goldcopd.org.
-
- Rodrigo GJ, Neffen H. A systematic review with meta-analysis of fluticasone furoate/vilanterol combination for the treatment of stable COPD. Pulm Pharmacol Ther. 2016;42:1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

