Long-term efficacy and safety of lamotrigine for all types of bipolar disorder
- PMID: 28360522
- PMCID: PMC5365320
- DOI: 10.2147/NDT.S128653
Long-term efficacy and safety of lamotrigine for all types of bipolar disorder
Abstract
Background: We investigated whether the long-term efficacy and safety of lamotrigine (LTG) for bipolar disorder (BP) differs between disease types (BP-I, BP-II, or BP not otherwise specified [BP-NOS]), and the efficacy of the concomitant use of antidepressants (ADs).
Methods: For >1 year, we observed 445 outpatients with BP (diagnosed by DSM-IV criteria) who initiated LTG treatment between July 1 and October 31, 2011, using the Himorogi Self-rating Depression (HSDS) and Anxiety Scales and the Clinical Global Impression-Improvement scale and also recorded adverse events.
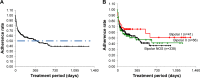
Results: Treatment efficacy was observed at week 4, with the improved HSDS scores sustained until week 52 for all types of BP; 50% of the patients with any type of BP could be treated with LTG for 1 year, whereas ~40% could be treated for >1.5 years. However, 25% of the patients were withdrawn within the first 4 weeks. The overall incidence of adverse events was 22.9% (104/455): 34.1% (14/41) for BP-I, 22.7% (15/66) for BP-II, and 22.2% (75/338) for BP-NOS. The most common adverse event was skin rash: 22.0% for BP-I, 16.7% for BP-II, and 12.1% for BP-NOS.
Limitations: There was no control group. Data were collected retrospectively.
Conclusion: With careful and adequate titration, long-term treatment with LTG is possible for any type of BP, with BP-NOS patients, the largest population in clinical practice, responding particularly well. Symptoms can improve with or without ADs. Large-scale prospective studies of the efficacy of ADs in bipolar treatment are warranted.
Keywords: antidepressant; anxiety; bipolar disorder; lamotrigine; long-term efficacy; skin rash.
Conflict of interest statement
Disclosure This study was funded by GlaxoSmithKline. The authors report no other conflicts of interest in this work.
Figures
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, Texas: American Psychiatric Publishing; 2013. DSM-5.
-
- Etain B, Lajnef M, Bellivier F, et al. Clinical expression of bipolar disorder type I as a function of age and polarity at onset: convergent findings in samples from France and the United States. J Clin Psychiatry. 2012;73(4):561–566. - PubMed
-
- Tondo L, Baldessarini RJ, Vázquez G, Lepri B, Visioli C. Clinical responses to antidepressants among 1036 acutely depressed patients with bipolar or unipolar major affective disorders. Acta Psychiatr Scand. 2013;127(5):355–364. - PubMed
-
- Undurraga J, Baldessarini RJ, Valentí M, et al. Bipolar depression: clinical correlates of receiving antidepressants. J Affect Disord. 2012;139(1):89–93. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

