Genetic Tracing of Cav3.2 T-Type Calcium Channel Expression in the Peripheral Nervous System
- PMID: 28360836
- PMCID: PMC5350092
- DOI: 10.3389/fnmol.2017.00070
Genetic Tracing of Cav3.2 T-Type Calcium Channel Expression in the Peripheral Nervous System
Abstract
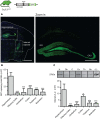
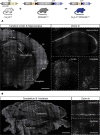
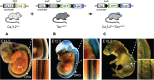
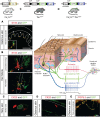
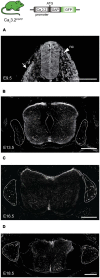
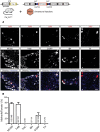
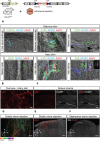
Characterizing the distinct functions of the T-type ion channel subunits Cav3.1, 3.2 or 3.3 has proven difficult due to their highly conserved amino-acid sequences and the lack of pharmacological blockers specific for each subunit. To precisely determine the expression pattern of the Cav3.2 channel in the nervous system we generated two knock-in mouse strains that express EGFP or Cre recombinase under the control of the Cav3.2 gene promoter. We show that in the brains of these animals, the Cav3.2 channel is predominantly expressed in the dentate gyrus of the hippocampus. In the peripheral nervous system, the activation of the promoter starts at E9.5 in neural crest cells that will give rise to dorsal root ganglia (DRG) neurons, but not sympathetic neurons. As development progresses the number of DRG cells expressing the Cav3.2 channel reaches around 7% of the DRG at E16.5, and remains constant until E18.5. Characterization of sensory neuron subpopulations at E18.5 showed that EGFP+ cells are a heterogeneous population consisting mainly of TrkB+ and TrkC+ cells, while only a small percentage of DRG cells were TrkA+. Genetic tracing of the sensory nerve end-organ innervation of the skin showed that the activity of the Cav3.2 channel promoter in sensory progenitors marks many mechanoreceptor and nociceptor endings, but spares slowly adapting mechanoreceptors with endings associated with Merkel cells. Our genetic analysis reveals for the first time that progenitors that express the Cav3.2 T-type calcium channel, defines a sensory specific lineage that populates a large proportion of the DRG. Using our Cav3.2-Cre mice together with AAV viruses containing a conditional fluorescent reporter (tdTomato) we could also show that Cre expression is largely restricted to two functionally distinct sensory neuron types in the adult ganglia. Cav3.2 positive neurons innervating the skin were found to only form lanceolate endings on hair follicles and are probably identical to D-hair receptors. A second population of nociceptive sensory neurons expressing the Cav3.2 gene was found to be positive for the calcitonin-gene related peptide but these neurons are deep tissue nociceptors that do not innervate the skin.
Keywords: Cav3.2; D-hair receptors; genetic tracing; hairy skin; muscle nociceptors; sensory neuron progenitors; slowly-adapting mechanoreceptors; spinal cord.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

