Saponins from Sanguisorba officinalis Improve Hematopoiesis by Promoting Survival through FAK and Erk1/2 Activation and Modulating Cytokine Production in Bone Marrow
- PMID: 28360858
- PMCID: PMC5353277
- DOI: 10.3389/fphar.2017.00130
Saponins from Sanguisorba officinalis Improve Hematopoiesis by Promoting Survival through FAK and Erk1/2 Activation and Modulating Cytokine Production in Bone Marrow
Abstract
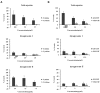
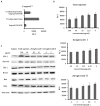
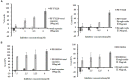
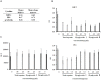
Radix Sanguisorbae, the root of Sanguisorba officinalis L. is used as traditional Chinese medicine. In recent decades, it has been reported to be clinically effective against myelosuppression induced by chemotherapy and/ or radiotherapy. However, the underlining mechanism has not been well studied. In this work, we evaluated the hematopoietic effect of total saponins from S. officinalis L. on myelosuppressive mice induced by cyclophosphamide and by60Co-γ-irradiation and confirmed the therapeutic effect. Then, we found total saponins and their characteristic constituents Ziyuglycoside I and Ziyuglycoside II can inhibit apoptosis of TF-1 cells caused by cytokine deprivation, and promote survival of mouse bone marrow nuclear cells through focal adhesion kinase (FAK) and extracellular signal-regulated kinase 1/2 (Erk1/2) activation in vitro. In addition, they can down-regulate macrophage inflammatory protein 2 (MIP-2), platelet factor 4 (PF4) and P-selectin secretion, which are reported to be suppressive to hematopoiesis, both in vitro and in vivo. These results suggest that promotion of survival through FAK and Erk1/2 activation and inhibition of suppressive cytokines in the bone marrow is likely to be the pharmacological mechanism underlying the hematopoietic effect of saponins from S. officinalis L.
Keywords: Sanguisorba officinalis L.; Ziyuglycoside I; Ziyuglycoside II; apoptosis; cytokine; hematopoiesis; myelosuppression; saponin.
Figures


Similar articles
-
Phytotherapeutic Activities of Sanguisorba officinalis and its Chemical Constituents: A Review.Am J Chin Med. 2018;46(2):299-318. doi: 10.1142/S0192415X18500155. Epub 2018 Feb 12. Am J Chin Med. 2018. PMID: 29433389 Review.
-
Antiangiogenic Effects of Ziyuglycoside II, a Major Active Compound of Sanguisorba officinalis L.Phytother Res. 2017 Sep;31(9):1449-1456. doi: 10.1002/ptr.5874. Epub 2017 Jul 18. Phytother Res. 2017. PMID: 28718964
-
Inhibitory effects of Sanguisorba officinalis root extract on HYBID (KIAA1199)-mediated hyaluronan degradation and skin wrinkling.Int J Cosmet Sci. 2019 Feb;41(1):12-20. doi: 10.1111/ics.12505. Epub 2019 Jan 9. Int J Cosmet Sci. 2019. PMID: 30485450 Clinical Trial.
-
Ginseng-Derived Panaxadiol Saponins Promote Hematopoiesis Recovery in Cyclophosphamide-Induced Myelosuppressive Mice: Potential Novel Treatment of Chemotherapy-Induced Cytopenias.Chin J Integr Med. 2018 Mar;24(3):200-206. doi: 10.1007/s11655-017-2754-8. Epub 2017 Apr 22. Chin J Integr Med. 2018. PMID: 28432529
-
Traditional Uses, Chemical Constituents and Biological Activities of Plants from the Genus Sanguisorba L.Am J Chin Med. 2017;45(2):199-224. doi: 10.1142/S0192415X17500136. Epub 2017 Mar 2. Am J Chin Med. 2017. PMID: 28249548 Review.
Cited by
-
Ca' Granda, Hortus simplicium: Restoring an Ancient Medicinal Garden of XV-XIX Century in Milan (Italy).Molecules. 2021 Nov 17;26(22):6933. doi: 10.3390/molecules26226933. Molecules. 2021. PMID: 34834025 Free PMC article.
-
Sanguisorbae Radix Suppresses Colorectal Tumor Growth Through PD-1/PD-L1 Blockade and Synergistic Effect With Pembrolizumab in a Humanized PD-L1-Expressing Colorectal Cancer Mouse Model.Front Immunol. 2021 Sep 29;12:737076. doi: 10.3389/fimmu.2021.737076. eCollection 2021. Front Immunol. 2021. PMID: 34659228 Free PMC article.
-
Sanguisorba officinalis L. derived from herbal medicine prevents intestinal inflammation by inducing autophagy in macrophages.Sci Rep. 2020 Jun 19;10(1):9972. doi: 10.1038/s41598-020-65306-4. Sci Rep. 2020. PMID: 32561763 Free PMC article.
-
Network Pharmacology-Based Investigation of the System-Level Molecular Mechanisms of the Hematopoietic Activity of Samul-Tang, a Traditional Korean Herbal Formula.Evid Based Complement Alternat Med. 2020 Feb 13;2020:9048089. doi: 10.1155/2020/9048089. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32104198 Free PMC article.
-
Rapid Profiling of Metabolites Combined with Network Pharmacology to Explore the Potential Mechanism of Sanguisorba officinalis L. against Thrombocytopenia.Metabolites. 2022 Nov 5;12(11):1074. doi: 10.3390/metabo12111074. Metabolites. 2022. PMID: 36355157 Free PMC article.
References
-
- American Society of Clinical Oncology (1994). Recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J. Clin. Oncol. 12 2471–2508. - PubMed
-
- Broxmeyer H. E., Cooper S., Hague N., Benninger L., Sarris A., Cornetta K., et al. (1995). Human chemokines: enhancement of specific activity and effects in vitro on normal and leukemic progenitors and a factor-dependent cell line and in vivo in mice. Ann. Hematol. 71 235–246. 10.1007/BF01744373 - DOI - PubMed
-
- Broxmeyer H. E., Sherry B., Cooper S., Lu L., Maze R., Beckmann M. P., et al. (1993). Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. Interacting effects involving suppression, synergistic suppression, and blocking of suppression. J. Immunol. 150 3448–3458. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

