Enhancement of Non-photochemical Quenching as an Adaptive Strategy under Phosphorus Deprivation in the Dinoflagellate Karlodinium veneficum
- PMID: 28360892
- PMCID: PMC5350143
- DOI: 10.3389/fmicb.2017.00404
Enhancement of Non-photochemical Quenching as an Adaptive Strategy under Phosphorus Deprivation in the Dinoflagellate Karlodinium veneficum
Abstract
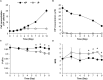
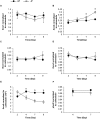
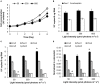
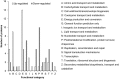
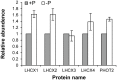
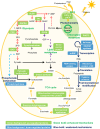
Intensified water column stratification due to global warming has the potential to decrease nutrient availability while increasing excess light for the photosynthesis of phytoplankton in the euphotic zone, which together will increase the need for photoprotective strategies such as non-photochemical quenching (NPQ). We investigated whether NPQ is enhanced and how it is regulated molecularly under phosphorus (P) deprivation in the dinoflagellate Karlodinium veneficum. We grew K. veneficum under P-replete and P-depleted conditions, monitored their growth rates and chlorophyll fluorescence, and conducted gene expression and comparative proteomic analyses. The results were used to characterize NPQ modulation and associated gene expression dynamics under P deprivation. We found that NPQ in K. veneficum was elevated significantly under P deprivation. Accordingly, the abundances of three light-harvesting complex stress-related proteins increased under P-depleted condition. Besides, many proteins related to genetic information flow were down-regulated while many proteins related to energy production and conversion were up-regulated under P deprivation. Taken together, our results indicate that K. veneficum cells respond to P deprivation by reconfiguring the metabolic landscape and up-tuning NPQ to increase the capacity to dissipate excess light energy and maintain the fluency of energy flow, which provides a new perspective about what adaptive strategy dinoflagellates have evolved to cope with P deprivation.
Keywords: dinoflagellates; energy flow; metabolic machinery reconfiguration; non-photochemical quenching; phosphorus deprivation.
Figures

Similar articles
-
The Dynamics of Energy Dissipation and Xanthophyll Conversion in Arabidopsis Indicate an Indirect Photoprotective Role of Zeaxanthin in Slowly Inducible and Relaxing Components of Non-photochemical Quenching of Excitation Energy.Front Plant Sci. 2017 Dec 8;8:2094. doi: 10.3389/fpls.2017.02094. eCollection 2017. Front Plant Sci. 2017. PMID: 29276525 Free PMC article.
-
Mesophyll-specific phytochromes impact chlorophyll light-harvesting complexes (LHCs) and non-photochemical quenching.Plant Signal Behav. 2019;14(7):1609857. doi: 10.1080/15592324.2019.1609857. Epub 2019 Apr 30. Plant Signal Behav. 2019. PMID: 31037997 Free PMC article.
-
A strain of the toxic dinoflagellate Karlodinium veneficum isolated from the East China Sea is an omnivorous phagotroph.Harmful Algae. 2020 Mar;93:101775. doi: 10.1016/j.hal.2020.101775. Epub 2020 Feb 24. Harmful Algae. 2020. PMID: 32307067
-
Photoprotection in cyanobacteria: regulation of light harvesting.Photochem Photobiol. 2008 Nov-Dec;84(6):1410-20. doi: 10.1111/j.1751-1097.2008.00453.x. Photochem Photobiol. 2008. PMID: 19067963 Review.
-
A New Light on Photosystem II Maintenance in Oxygenic Photosynthesis.Front Plant Sci. 2019 Jul 31;10:975. doi: 10.3389/fpls.2019.00975. eCollection 2019. Front Plant Sci. 2019. PMID: 31417592 Free PMC article. Review.
Cited by
-
RNA-seq Insights Into the Impact of Alteromonas macleodii on Isochrysis galbana.Front Microbiol. 2021 Sep 8;12:711998. doi: 10.3389/fmicb.2021.711998. eCollection 2021. Front Microbiol. 2021. PMID: 34566917 Free PMC article.
-
Distinct iron acquisition strategies in oceanic and coastal variants of the mixotrophic dinoflagellate Karlodinium.ISME J. 2025 Jan 2;19(1):wraf099. doi: 10.1093/ismejo/wraf099. ISME J. 2025. PMID: 40391772 Free PMC article.
-
Age-Specific Physiological Adjustments of Spirodela polyrhiza to Sulfur Deficiency.Plants (Basel). 2025 Jun 20;14(13):1907. doi: 10.3390/plants14131907. Plants (Basel). 2025. PMID: 40647915 Free PMC article.
-
Nutrient Deficiencies Impact on the Cellular and Metabolic Responses of Saxitoxin Producing Alexandrium minutum: A Transcriptomic Perspective.Mar Drugs. 2023 Sep 18;21(9):497. doi: 10.3390/md21090497. Mar Drugs. 2023. PMID: 37755110 Free PMC article.
-
Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition.Biology (Basel). 2021 Oct 18;10(10):1060. doi: 10.3390/biology10101060. Biology (Basel). 2021. PMID: 34681157 Free PMC article. Review.
References
-
- Ambarsari I., Brown B. E., Barlow R. G., Britton G., Cummings D. (1997). Fluctuations in algal chlorophyll and carotenoid pigments during solar bleaching in the coral Goniastrea aspera at Phuket, Thailand. Mar. Ecol. Prog. Ser. 159 303–307. 10.3354/meps159303 - DOI
-
- Anderson B., Barber J. (1996). “Mechanisms of photodamage and protein degradation during photoinhibition of photosystem II,” in Photosynthesis and the Environment ed. Baker N. R. (Dordrecht: Springer; ) 10.1007/0-306-48135-9_4 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

