Fasciola hepatica Immune Regulates CD11c+ Cells by Interacting with the Macrophage Gal/GalNAc Lectin
- PMID: 28360908
- PMCID: PMC5350155
- DOI: 10.3389/fimmu.2017.00264
Fasciola hepatica Immune Regulates CD11c+ Cells by Interacting with the Macrophage Gal/GalNAc Lectin
Abstract
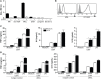
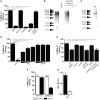
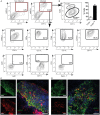
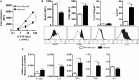
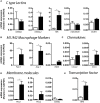
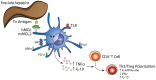
Fasciolosis, caused by Fasciola hepatica and Fasciola gigantica, is a trematode zoonosis of interest in public health and livestock production. Like other helminths, F. hepatica modulates the host immune response by inducing potent polarized Th2 and regulatory T cell immune responses and by downregulating the production of Th1 cytokines. In this work, we show that F. hepatica glycans increase Th2 immune responses by immunomodulating TLR-induced maturation and function of dendritic cells (DCs). This process was mediated by the macrophage Gal/GalNAc lectin (MGL) expressed on DCs, which recognizes the Tn antigen (GalNAc-Ser/Thr) on parasite components. More interestingly, we identified MGL-expressing CD11c+ cells in infected animals and showed that these cells are recruited both to the peritoneum and the liver upon F. hepatica infection. These cells express the regulatory cytokines IL-10, TNFα and TGFβ and a variety of regulatory markers. Furthermore, MGL+ CD11c+ cells expand parasite-specific Th2/regulatory cells and suppress Th1 polarization. The results presented here suggest a potential role of MGL in the immunomodulation of DCs induced by F. hepatica and contribute to a better understanding of the molecular and immunoregulatory mechanisms induced by this parasite.
Keywords: C-type lectin receptors; dendritic cell; glycans; helminth; immune regulation; macrophage Gal/GalNAc lectin.
Figures



References
-
- Dowling DJ, Hamilton CM, Donnelly S, La Course J, Brophy PM, Dalton J, et al. Major secretory antigens of the helminth Fasciola hepatica activate a suppressive dendritic cell phenotype that attenuates Th17 cells but fails to activate Th2 immune responses. Infect Immun (2010) 78(2):793–801.10.1128/IAI.00573-09 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

