Macrophage type modulates osteogenic differentiation of adipose tissue MSCs
- PMID: 28361303
- PMCID: PMC5552848
- DOI: 10.1007/s00441-017-2598-8
Macrophage type modulates osteogenic differentiation of adipose tissue MSCs
Abstract
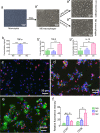
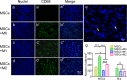
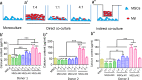
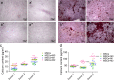
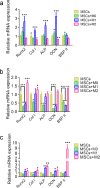
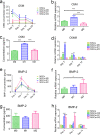
Since the reconstruction of large bone defects remains a challenge, knowledge about the biology of bone healing is desirable to develop novel strategies for improving the treatment of bone defects. In osteoimmunology, macrophages are the central component in the early stage of physiological response after bone injury and bone remodeling in the late stage. During this process, a switch of macrophage phenotype from pro-inflammatory (M1) to anti-inflammatory (M2) is observed. An appealing option for bone regeneration would be to exploit this regulatory role for the benefit of osteogenic differentiation of osteoprogenitor cells (e.g., mesenchymal stem cells; MSCs) and to eventually utilize this knowledge to improve the therapeutic outcome of bone regenerative treatment. In view of this, we focused on the in vitro interaction of different macrophage subtypes with adipose tissue MSCs to monitor the behavior (i.e. proliferation, differentiation and mineralization) of the latter in dedicated co-culture models. Our data show that co-culture of MSCs with M2 macrophages, but not with M1 macrophages or M0 macrophages, results in significantly increased MSC mineralization caused by soluble factors. Specifically, M2 macrophages promoted the proliferation and osteogenic differentiation of MSCs, while M0 and M1 macrophages solely stimulated the osteogenic differentiation of MSCs in the early and middle stages during co-culture. Secretion of the soluble factors oncostatin M (OSM) and bone morphogenetic protein 2 (BMP-2) by macrophages showed correlation with MSC gene expression levels for OSM-receptor and BMP-2, suggesting the involvement of both signaling pathways in the osteogenic differentiation of MSCs.
Keywords: Cell culture model; MSC; Macrophage; Osteogenic differentiation.
Conflict of interest statement
Conflict of interest
The authors have no conflict of interest to disclose.
Funding
This study was financially supported by The Netherlands Organization for Health Research and Development (ZonMw, project number 40-41400-98-1401) and China Scholarship Council (No. 2010622061).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

