Sustained delivery of calcium and orthophosphate ions from amorphous calcium phosphate and poly(L-lactic acid)-based electrospinning nanofibrous scaffold
- PMID: 28361908
- PMCID: PMC5374505
- DOI: 10.1038/srep45655
Sustained delivery of calcium and orthophosphate ions from amorphous calcium phosphate and poly(L-lactic acid)-based electrospinning nanofibrous scaffold
Abstract
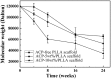
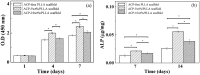
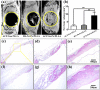
The purpose of this study is to investigate electrospinning poly(L-lactic acid) (PLLA) nanofibrous scaffold with different contents of amorphous calcium phosphate (ACP), which is suitable for using in bone regeneration through sustained release of calcium and orthophosphate ions. Three groups of nanofibrous scaffolds, ACP-free PLLA, ACP-5 wt%/PLLA and ACP-10 wt%/PLLA, are developed and characterized by scanning electron microscopy and gel permeation chromatography. Calcium and phosphate colorimetric assay kits are used to test ions released from scaffold during hydrolytic degradation. The results show ACP-5 wt%/PLLA and ACP-10 wt%/PLLA scaffolds have relatively high degradation rates than ACP-free PLLA group. The bioactivity evaluation further reveals that ACP-5 wt%/PLLA scaffold presents more biocompatible feature with pre-osteoblast cells and significant osteogenesis ability of calvarial bone defect. Due to the facile preparation method, sustained calcium and orthophosphate release behavior, and excellent osteogenesis capacity, the presented ACP/PLLA nanofibrous scaffold has potential applications in bone tissue engineering.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Cui F. Z., Li Y. & Ge J. Self-assembly of mineralized collagen composites. Mat. Sci. Eng. R 57, 1–27 (2007).
-
- Niu X. F., Feng Q. L., Wang M. B., Guo X. D. & Zheng Q. X. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 134, 111–117 (2009). - PubMed
-
- Olszta M. J. et al. Bone structure and formation: A new perspective. Mat. Sci. Eng. R 58, 77–116 (2007).
-
- Wegst U. G. K., Bai H., Saiz E., Tomsia A. P. & Ritchie R. O. Bioinspired structural materials. Nat. Mater. 14, 23–36 (2015). - PubMed
-
- Huang Y. et al. Effects of hydroxyapatite/collagen composite on osteogenic differentiation of rat bone marrow derived mesenchymal stem cells. J. Compos. Mater. 48, 1971–1980 (2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

