Ash aggregation enhanced by deposition and redistribution of salt on the surface of volcanic ash in eruption plumes
- PMID: 28361966
- PMCID: PMC5374634
- DOI: 10.1038/srep45762
Ash aggregation enhanced by deposition and redistribution of salt on the surface of volcanic ash in eruption plumes
Abstract
Interactions with volcanic gases in eruption plumes produce soluble salt deposits on the surface of volcanic ash. While it has been postulated that saturation-driven precipitation of salts following the dissolution of ash surfaces by condensed acidic liquids is a primary mechanism of salt formation during an eruption, it is only recently that this mechanism has been subjected to detailed study. Here we spray water and HCl droplets into a suspension of salt-doped synthetic glass or volcanic ash particles, and produce aggregates. Deposition of acidic liquid droplets on ash particles promotes dissolution of existing salts and leaches cations from the underlying material surface. The flow of liquid, due to capillary forces, will be directed to particle-particle contact points where subsequent precipitation of salts will cement the aggregate. Our data suggest that volcanically-relevant loads of surface salts can be produced by acid condensation in eruptive settings. Several minor and trace elements mobilised by surface dissolution are biologically relevant; geographic areas with aggregation-mediated ash fallout could be "hotspots" for the post-deposition release of these elements. The role of liquids in re-distributing surface salts and cementing ash aggregates also offers further insight into the mechanisms which preserve well-structured aggregates in some ash deposits.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
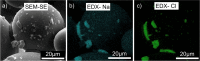

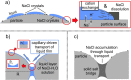
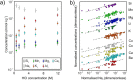
References
-
- Witham C. S., Oppenheimer C. & Horwell C. J. Volcanic ash-leachates: a review and recommendations for sampling methods. Journal of Volcanology and Geothermal Research. 141, 299–326 (2004).
-
- Ayris P. M. & Delmelle P. The immediate environmental effects of tephra emission. Bull. Volcanol. 74, 1905–1936 (2012).
-
- Gilbert J. S. & Lane S. J. The origin of accretionary lapilli. Bull. Volcanol. 56, 398–411 (1994).
-
- Mueller S. B., Kueppers U., Ayris P. M., Jacob M. & Dingwell D. B. Experimental volcanic ash aggregation: Internal structuring of accretionary lapilli and the role of liquid bonding. Earth Planet. Sci. Lett. 433, 232–240 (2016).
-
- Ayris P. M. et al.. HCl uptake by volcanic ash in the high temperature eruption plume: mechanistic insights. Geochim. Cosmochim. Acta. 144, 188–201 (2014).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

