Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with tenofovir disoproxil fumarate in France: a prospective cohort study
- PMID: 28362068
- PMCID: PMC5467614
- DOI: 10.7448/IAS.20.1.21426
Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with tenofovir disoproxil fumarate in France: a prospective cohort study
Abstract
Introduction: Long-term tenofovir disoproxil fumarate (TDF) use has been associated with significant regression of liver fibrosis during hepatitis B virus (HBV) mono-infection, yet little is known during HIV-HBV coinfection. The aim of this study was to evaluate the evolution of liver fibrosis and its determinants in TDF-treated coinfected patients.
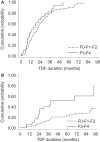
Methods: In this prospective cohort study, 167 HIV-HBV-infected patients initiating TDF-containing antiretroviral therapy were included. Fibrosis was assessed using the FibroTest® at baseline and every six to twelve months. Risk factors for fibrosis progression (F0-F1-F2 to F3-F4) and regression (F3-F4 to F0-F1-F2) were evaluated.
Results: At baseline, 134 (80.2%) patients had detectable HBV-DNA (median = 4.93 log10 IU/mL, IQR = 2.94-7.15) and 104 (62.3%) had hepatitis B "e" antigen-positive serology. Median follow-up was sixty months (IQR = 36-93). In the 47 (28.1%) patients with F3-F4 baseline fibrosis, 7/47 (14.9%) regressed to F0-F1-F2 at last follow-up visit. Fibrosis regression was significantly associated with higher CD4+ cell counts (P = 0.009) and lower fasting triglyceride levels (P = 0.007) at TDF-initiation. In the 120 (71.9%) patients with F0-F1-F2-baseline fibrosis, 20/120 (16.7%) progressed to F3-F4 at last follow-up visit. Fibrosis progression was associated with male gender (P = 0.01), older age (P = 0.001), from low/moderate HBV-endemic country (P = 0.007), lower nadir CD4+ cell count (P = 0.03), higher fasting glycaemia (P = 0.03) and anaemia (P = 0.004) at TDF-initiation. Control of HBV replication at end of follow-up was extensive (88.1%), while no HBV-related factors emerged as predictors of progression/regression. Incidence of severe liver-related events was low (n = 4, rate = 0.5/100 person-years).
Conclusion: Liver fibrosis levels are stable for most coinfected patients undergoing TDF, despite control of HBV replication. Nevertheless, a concerning amount of liver fibrosis progression did occur, which could be partly explained by metabolic abnormalities and past severe immunosuppression and requires further evaluation.
Keywords: hepatocellular carcinoma; immunosuppression; liver cirrhosis; liver fibrosis; noninvasive markers.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
References
-
- Chen C-J, Yang H-I, Iloeje UH. REVEAL-HBV Study Group . Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009. May;49(5 Suppl):S72–12. - PubMed
-
- Kitrinos KM, Corsa A, Liu Y, Flaherty J, Snow-Lampart A, Marcellin P, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014. February;59(2):434–42. - PubMed
-
- Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013. February 9;381(9865):468–75. - PubMed
-
- Papatheodoridis GV, Chan HL-Y, Hansen BE, Janssen HLA, Lampertico P.. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015. April;62(4):956–67. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

