Immunogenicity and safety evaluation of bivalent types 1 and 3 oral poliovirus vaccine by comparing different poliomyelitis vaccination schedules in China: A randomized controlled non-inferiority clinical trial
- PMID: 28362135
- PMCID: PMC5489289
- DOI: 10.1080/21645515.2017.1288769
Immunogenicity and safety evaluation of bivalent types 1 and 3 oral poliovirus vaccine by comparing different poliomyelitis vaccination schedules in China: A randomized controlled non-inferiority clinical trial
Abstract
Background: The type 2 component of the oral poliovirus vaccine is targeted for global withdrawal through a switch from the trivalent oral poliovirus vaccine (tOPV) to a bivalent oral poliovirus vaccine (bOPV). The switch is intended to prevent paralytic polio caused by circulating vaccine-derived poliovirus type 2. We aimed to assess the immunogenicity and safety profile of 6 vaccination schedules with different sequential doses of inactivated poliovirus vaccine (IPV), tOPV, or bOPV.
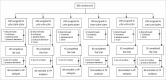
Methods: A randomized controlled trial was conducted in China in 2015. Healthy newborn babies randomly received one of the following 6 vaccination schedules: cIPV-bOPV-bOPV(I-B-B), cIPV-tOPV-tOPV(I-T-T), cIPV-cIPV-bOPV(I-I-B), cIPV-cIPV-tOPV(I-I-T), cIPV-cIPV-cIPV(I-I-I), or tOPV-tOPV-tOPV(T-T-T). Doses were administered sequentially at 4-6 week intervals after collecting baseline blood samples. Patients were proactively followed up for observation of adverse events after the first dose and 30 days after all doses. The primary study objective was to investigate the immunogenicity and safety profile of different vaccine schedules, evaluated by seroconversion, seroprotection and antibody titer against poliovirus types 1, 2, and 3 in the per-protocol population.
Results: Of 600 newborn babies enrolled, 504 (84.0%) were included in the per-protocol population. For type 1 poliovirus, the differences in the seroconversion were 1.17% (95% CI = -2.74%, 5.08%) between I-B-B and I-T-T and 0.00% (95% CI: -6.99%, 6.99%) between I-I-B and I-I-T; for type 3 poliovirus, differences in the seroconversion were 3.49% (95% CI: -1.50%, 8.48%) between I-B-B and I-T-T and -2.32% (95% CI: -5.51%, 0.86%) between I-I-B and I-I-T. The non-inferiority conclusion was achieved in both poliovirus type 1 and 3 with the margin of -10%. Of 24 serious adverse events reported, no one was vaccine-related.
Conclusions: The vaccination schedules with bOPV followed by one or 2 doses of IPV were recommended to substitute for vaccinations involving tOPV without compromising the immunogenicity and safety in the Chinese population. The findings will be essential for policy formulation by national and global authorities to facilitate polio elimination.
Keywords: immunization; inactivated polio vaccine; infectious disease; oral polio vaccine; polio; poliomyelitis; poliovirus; vaccine; vaccine schedule.
Figures


Similar articles
-
Immunogenicity of New Primary Immunization Schedules With Inactivated Poliovirus Vaccine and Bivalent Oral Polio Vaccine for the Polio Endgame: A Review.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S35-S41. doi: 10.1093/cid/ciy633. Clin Infect Dis. 2018. PMID: 30376081 Free PMC article. Review.
-
Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study.Lancet Infect Dis. 2015 Nov;15(11):1273-82. doi: 10.1016/S1473-3099(15)00219-4. Epub 2015 Aug 26. Lancet Infect Dis. 2015. PMID: 26318714 Clinical Trial.
-
Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial.Lancet. 2015 Dec 12;386(10011):2413-21. doi: 10.1016/S0140-6736(15)00237-8. Epub 2015 Sep 18. Lancet. 2015. PMID: 26388534 Clinical Trial.
-
Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open-label randomised controlled trial.Lancet. 2016 Jul 9;388(10040):158-69. doi: 10.1016/S0140-6736(16)00703-0. Epub 2016 May 19. Lancet. 2016. PMID: 27212429 Clinical Trial.
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
Cited by
-
Mannose and Lactobionic Acid in Nasal Vaccination: Enhancing Antigen Delivery via C-Type Lectin Receptors.Pharmaceutics. 2024 Oct 8;16(10):1308. doi: 10.3390/pharmaceutics16101308. Pharmaceutics. 2024. PMID: 39458637 Free PMC article. Review.
-
Assessing the mucosal intestinal and systemic humoral immunity of sequential schedules of inactivated poliovirus vaccine and bivalent oral poliovirus vaccine for essential immunization in Bangladesh: An open-label, randomized controlled trial.Vaccine. 2024 Sep 17;42(22):126216. doi: 10.1016/j.vaccine.2024.126216. Epub 2024 Aug 14. Vaccine. 2024. PMID: 39146859 Free PMC article. Clinical Trial.
-
Immunogenicity of sequential poliovirus vaccination schedules with different strains of poliomyelitis vaccines in Chongqing, China: a cross-sectional survey.Hum Vaccin Immunother. 2021 Jul 3;17(7):2125-2131. doi: 10.1080/21645515.2020.1868269. Epub 2021 Mar 24. Hum Vaccin Immunother. 2021. PMID: 33759702 Free PMC article.
-
Cost-effectiveness of various immunization schedules with inactivated Sabin strain polio vaccine in Hangzhou, China.Front Public Health. 2022 Sep 23;10:990042. doi: 10.3389/fpubh.2022.990042. eCollection 2022. Front Public Health. 2022. PMID: 36211670 Free PMC article.
-
Immunogenicity of New Primary Immunization Schedules With Inactivated Poliovirus Vaccine and Bivalent Oral Polio Vaccine for the Polio Endgame: A Review.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S35-S41. doi: 10.1093/cid/ciy633. Clin Infect Dis. 2018. PMID: 30376081 Free PMC article. Review.
References
-
- Kew OM, Sutter RW, De Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Ann Rev Microbiol. 2005; 59:587-635; https://doi.org/10.1146/annurev.micro.58.030603.123625 - DOI - PubMed
-
- Global Polio Eradication Initiative (GPEI) Polio Eradication and Endgame Strategic Plan: 2013–2018. 2013. Available at: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf
-
- Global Polio Eradication Initiative (GPEI) Wild poliovirus list. n.d.. Available at: http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/
-
- Garon JR and Orenstein WA. A worldwide shift in polio vaccines for routine immunization. Lancet 2015; 386(10011):2375-77; PMID:26388533; https://doi.org/10.1016/S0140-6736(15)00243-3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
