The non-conserved region of MRP is involved in the virulence of Streptococcus suis serotype 2
- PMID: 28362221
- PMCID: PMC5711419
- DOI: 10.1080/21505594.2017.1313373
The non-conserved region of MRP is involved in the virulence of Streptococcus suis serotype 2
Abstract
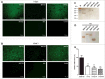
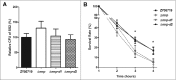
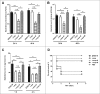
Muramidase-released protein (MRP) of Streptococcus suis serotype 2 (SS2) is an important epidemic virulence marker with an unclear role in bacterial infection. To investigate the biologic functions of MRP, 3 mutants named Δmrp, Δmrp domain 1 (Δmrp-d1), and Δmrp domain 2 (Δmrp-d2) were constructed to assess the phenotypic changes between the parental strain and the mutant strains. The results indicated that MRP domain 1 (MRP-D1, the non-conserved region of MRP from a virulent strain, a.a. 242-596) played a critical role in adherence of SS2 to host cells, compared with MRP domain 1* (MRP-D1*, the non-conserved region of MRP from a low virulent strain, a.a. 239-598) or MRP domain 2 (MRP-D2, the conserved region of MRP, a.a. 848-1222). We found that MRP-D1 but not MRP-D2, could bind specifically to fibronectin (FN), factor H (FH), fibrinogen (FG), and immunoglobulin G (IgG). Additionally, we confirmed that mrp-d1 mutation significantly inhibited bacteremia and brain invasion in a mouse infection model. The mrp-d1 mutation also attenuated the intracellular survival of SS2 in RAW246.7 macrophages, shortened the growth ability in pig blood and decreased the virulence of SS2 in BALB/c mice. Furthermore, antiserum against MRP-D1 was found to dramatically impede SS2 survival in pig blood. Finally, immunization with recombinant MRP-D1 efficiently enhanced murine viability after SS2 challenge, indicating its potential use in vaccination strategies. Collectively, these results indicated that MRP-D1 is involved in SS2 virulence and eloquently demonstrate the function of MRP in pathogenesis of infection.
Keywords: Streptococcus suis serotype 2; infection; muramidase-released protein; vaccine; virulence.
Figures




Comment in
-
Muramidase-released protein of Streptococcus suis: New insight into its impact on virulence.Virulence. 2017 Oct 3;8(7):1078-1080. doi: 10.1080/21505594.2017.1325985. Epub 2017 May 3. Virulence. 2017. PMID: 28467144 Free PMC article. No abstract available.
References
-
- Gottschalk M, Segura M. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet Microbiol 2000; 76:259-72; PMID:10973700; http://dx.doi.org/ 10.1016/S0378-1135(00)00250-9 - DOI - PubMed
-
- Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Commun 1997; 21:381-407; PMID:9266659; http://dx.doi.org/ 10.1023/A:1005870317757 - DOI - PubMed
-
- Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol 2012; 7:259-79; PMID:22324994; http://dx.doi.org/ 10.2217/fmb.11.149 - DOI - PubMed
-
- Feng Y, Zhang H, Wu Z, Wang S, Cao M, Hu D, Wang C.. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence 2014; 5:477-97; PMID:24667807; http://dx.doi.org/ 10.4161/viru.28595 - DOI - PMC - PubMed
-
- Berthelot-Herault F, Gottschalk M, Morvan H, Kobisch M. Dilemma of virulence of Streptococcus suis: Canadian isolate 89–1591 characterized as a virulent strain using a standardized experimental model in pigs. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire 2005; 69:236-40; PMID:16187555 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
