Establishing a small animal model for evaluating protective immunity against mumps virus
- PMID: 28362871
- PMCID: PMC5375130
- DOI: 10.1371/journal.pone.0174444
Establishing a small animal model for evaluating protective immunity against mumps virus
Abstract
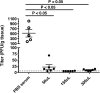
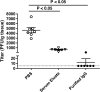
Although mumps vaccines have been used for several decades, protective immune correlates have not been defined. Recently, mumps outbreaks have occurred in vaccinated populations. To better understand the causes of the outbreaks and to develop means to control outbreaks in mumps vaccine immunized populations, defining protective immune correlates will be critical. Unfortunately, no small animal model for assessing mumps immunity exists. In this study, we evaluated use of type I interferon (IFN) alpha/beta receptor knockout mice (IFN-α/βR-/-) for such a model. We found these mice to be susceptible to mumps virus administered intranasally and intracranially. Passive transfer of purified IgG from immunized mice protected naïve mice from mumps virus infection, confirming the role of antibody in protection and demonstrating the potential for this model to evaluate mumps immunity.
Conflict of interest statement
Figures


References
-
- Carbone KM, Wolinsky JS. 2001. Mumps virus, p. 1381–1441. In Knipe DM, Howley PM (eds.), Fields Virology 4th Ed. Lippincott Williams and Wilkins, New York.
-
- Philip RN, Reinhard KR, Lackman DB. 1995. Observations on a mumps epidemic in a “virgin” population. 1958. Am. J. Epidemiol. 142:233–253. - PubMed
-
- Kaaijk P, van der Zeijst B, Boog M, Hoitink C. 2008. Increased mumps incidence in the Netherlands: review on the possible role of vaccine strain and genotype. Euro Surveill. 13:6–8. - PubMed
-
- Pagano JS, Levine RH, Sugg WC, Finger JA. 1967. Clinical trial of new attenuated mumps virus vaccine (Jeryl Lynn strain): preliminary report. Prog. Immunobiol. Stand. 3:196–202. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

