Structural principles controlling HIV envelope glycosylation
- PMID: 28363124
- PMCID: PMC5513759
- DOI: 10.1016/j.sbi.2017.03.008
Structural principles controlling HIV envelope glycosylation
Abstract
The heavily glycosylated, trimeric HIV-1 envelope (Env) protein is the sole viral protein exposed on the HIV-1 virion surface and is thus a main focus of antibody-mediated vaccine development. Dense glycosylation at the outer domain of Env constrains normal enzymatic processing, stalling the glycans at immature oligomannose-type structures. Furthermore, native trimerization imposes additional steric constraints, which generate an extensive 'trimer-induced mannose patch'. Importantly, the immature glycans present a highly conserved feature of the virus that is targeted by broadly neutralizing antibodies. Quantitative mass spectrometry of glycopeptides together with structures of the trimeric viral-spike define the steric principles controlling processing and provide a detailed map of the glycan shield.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


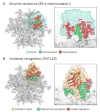
References
-
- Lasky LA, Groopman JE, Fennie CW, Benz PM, Capon DJ, Dowbenko DJ, Nakamura GR, Nunes WM, Renz ME, Berman PW. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science. 1986;233:209–212. - PubMed
-
- Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. - PubMed
-
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

