Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells
- PMID: 28363897
- PMCID: PMC5413338
- DOI: 10.1084/jem.20161533
Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells
Abstract
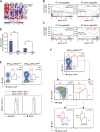
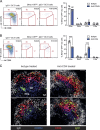
Plasma cells (PCs) derived from germinal centers (GCs) secrete the high-affinity antibodies required for long-term serological immunity. Nevertheless, the process whereby GC B cells differentiate into PCs is uncharacterized, and the mechanism underlying the selective PC differentiation of only high-affinity GC B cells remains unknown. In this study, we show that differentiation into PCs is induced among a discrete subset of high-affinity B cells residing within the light zone of the GC. Initiation of differentiation required signals delivered upon engagement with intact antigen. Signals delivered by T follicular helper cells were not required to initiate differentiation but were essential to complete the differentiation process and drive migration of maturing PCs through the dark zone and out of the GC. This bipartite or two-signal mechanism has likely evolved to both sustain protective immunity and avoid autoantibody production.
© 2017 Kräutler et al.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

