CD137 Plays Both Pathogenic and Protective Roles in Type 1 Diabetes Development in NOD Mice
- PMID: 28363905
- PMCID: PMC5426805
- DOI: 10.4049/jimmunol.1601851
CD137 Plays Both Pathogenic and Protective Roles in Type 1 Diabetes Development in NOD Mice
Abstract
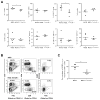
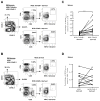
We previously reported that CD137 (encoded by Tnfrsf9) deficiency suppressed type 1 diabetes (T1D) progression in NOD mice. We also demonstrated that soluble CD137 produced by regulatory T cells contributed to their autoimmune-suppressive function in this model. These results suggest that CD137 can either promote or suppress T1D development in NOD mice depending on where it is expressed. In this study, we show that NOD.Tnfrsf9-/- CD8 T cells had significantly reduced diabetogenic capacity, whereas absence of CD137 in non-T and non-B cells had a limited impact on T1D progression. In contrast, NOD.Tnfrsf9-/- CD4 T cells highly promoted T1D development. We further demonstrated that CD137 was important for the accumulation of β cell-autoreactive CD8 T cells but was dispensable for their activation in pancreatic lymph nodes. The frequency of islet-infiltrating CD8 T cells was reduced in NOD.Tnfrsf9-/- mice in part because of their decreased proliferation. Furthermore, CD137 deficiency did not suppress T1D development in NOD mice expressing the transgenic NY8.3 CD8 TCR. This suggests that increased precursor frequency of β cell-autoreactive CD8 T cells in NY8.3 mice obviated a role for CD137 in diabetogenesis. Finally, blocking CD137-CD137 ligand interaction significantly delayed T1D onset in NOD mice. Collectively, our results indicate that one important diabetogenic function of CD137 is to promote the expansion and accumulation of β cell-autoreactive CD8 T cells, and in the absence of CD137 or its interaction with CD137 ligand, T1D progression is suppressed.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures








Similar articles
-
The CD137 Ligand Is Important for Type 1 Diabetes Development but Dispensable for the Homeostasis of Disease-Suppressive CD137+ FOXP3+ Regulatory CD4 T Cells.J Immunol. 2020 Jun 1;204(11):2887-2899. doi: 10.4049/jimmunol.1900485. Epub 2020 Apr 15. J Immunol. 2020. PMID: 32295876 Free PMC article.
-
Recombinant soluble CD137 prevents type one diabetes in nonobese diabetic mice.J Autoimmun. 2013 Dec;47:94-103. doi: 10.1016/j.jaut.2013.09.002. Epub 2013 Oct 18. J Autoimmun. 2013. PMID: 24145149
-
The B10 Idd9.3 locus mediates accumulation of functionally superior CD137(+) regulatory T cells in the nonobese diabetic type 1 diabetes model.J Immunol. 2012 Nov 15;189(10):5001-15. doi: 10.4049/jimmunol.1101013. Epub 2012 Oct 12. J Immunol. 2012. PMID: 23066155 Free PMC article.
-
Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice.Ann N Y Acad Sci. 2005 Jun;1051:72-87. doi: 10.1196/annals.1361.048. Ann N Y Acad Sci. 2005. PMID: 16126946 Review.
-
Bridging Mice to Men: Using HLA Transgenic Mice to Enhance the Future Prediction and Prevention of Autoimmune Type 1 Diabetes in Humans.Methods Mol Biol. 2016;1438:137-51. doi: 10.1007/978-1-4939-3661-8_9. Methods Mol Biol. 2016. PMID: 27150089 Review.
Cited by
-
CD70 Inversely Regulates Regulatory T Cells and Invariant NKT Cells and Modulates Type 1 Diabetes in NOD Mice.J Immunol. 2020 Oct 1;205(7):1763-1777. doi: 10.4049/jimmunol.2000148. Epub 2020 Aug 31. J Immunol. 2020. PMID: 32868408 Free PMC article.
-
Mechanisms and therapeutic strategies of immune checkpoint molecules and regulators in type 1 diabetes.Front Endocrinol (Lausanne). 2023 Jan 10;13:1090842. doi: 10.3389/fendo.2022.1090842. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36704045 Free PMC article. Review.
-
The long and winding road: From mouse linkage studies to a novel human therapeutic pathway in type 1 diabetes.Front Immunol. 2022 Jul 22;13:918837. doi: 10.3389/fimmu.2022.918837. eCollection 2022. Front Immunol. 2022. PMID: 35935980 Free PMC article. Review.
-
Combined congenic mapping and nuclease-based gene targeting for studying allele-specific effects of Tnfrsf9 within the Idd9.3 autoimmune diabetes locus.Sci Rep. 2019 Mar 13;9(1):4316. doi: 10.1038/s41598-019-40898-8. Sci Rep. 2019. PMID: 30867509 Free PMC article.
-
The Role of NOD Mice in Type 1 Diabetes Research: Lessons from the Past and Recommendations for the Future.Front Endocrinol (Lausanne). 2018 Feb 23;9:51. doi: 10.3389/fendo.2018.00051. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29527189 Free PMC article.
References
-
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. - PubMed
-
- Jang IK, Lee ZH, Kim YJ, Kim SH, Kwon BS. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-kappa B. Biochemical and biophysical research communications. 1998;242:613–620. - PubMed
-
- Cannons JL, Hoeflich KP, Woodgett JR, Watts TH. Role of the stress kinase pathway in signaling via the T cell costimulatory receptor 4-1BB. Journal of immunology. 1999;163:2990–2998. - PubMed
-
- Cannons JL, Choi Y, Watts TH. Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. Journal of immunology. 2000;165:6193–6204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

