Alcohol Dependence Disrupts Amygdalar L-Type Voltage-Gated Calcium Channel Mechanisms
- PMID: 28363981
- PMCID: PMC5413190
- DOI: 10.1523/JNEUROSCI.3721-16.2017
Alcohol Dependence Disrupts Amygdalar L-Type Voltage-Gated Calcium Channel Mechanisms
Abstract
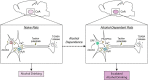
L-type voltage-gated calcium channels (LTCCs) are implicated in several psychiatric disorders that are comorbid with alcoholism and involve amygdala dysfunction. Within the amygdala, the central nucleus (CeA) is critical in acute alcohol's reinforcing actions, and its dysregulation in human alcoholics drives their negative emotional state and motivation to drink. Here we investigated the specific role of CeA LTCCs in the effects of acute alcohol at the molecular, cellular physiology, and behavioral levels, and their potential neuroadaptation in alcohol-dependent rats. Alcohol increases CeA activity (neuronal firing rates and GABA release) in naive rats by engaging LTCCs, and intra-CeA LTCC blockade reduces alcohol intake in nondependent rats. Alcohol dependence reduces CeA LTCC membrane abundance and disrupts this LTCC-based mechanism; instead, corticotropin-releasing factor type 1 receptors (CRF1s) mediate alcohol's effects on CeA activity and drive the escalated alcohol intake of alcohol-dependent rats. Collectively, our data indicate that alcohol dependence functionally alters the molecular mechanisms underlying the CeA's response to alcohol (from LTCC- to CRF1-driven). This mechanistic switch contributes to and reflects the prominent role of the CeA in the negative emotional state that drives excessive drinking.SIGNIFICANCE STATEMENT The central amygdala (CeA) plays a critical role in the development of alcohol dependence. As a result, much preclinical alcohol research aims to identify relevant CeA neuroadaptions that promote the transition to dependence. Here we report that acute alcohol increases CeA neuronal activity in naive rats by engaging L-type calcium channels (LTCCs) and that intra-CeA LTCC blockade reduces alcohol intake in nondependent rats. Alcohol dependence disrupts this LTCC-based mechanism; instead, corticotropin-releasing factor type 1 receptors (CRF1s) mediate alcohol's effects on CeA activity and drive the escalated alcohol intake of alcohol-dependent rats. This switch reflects the important role of the CeA in the pathophysiology of alcohol dependence and represents a new potential avenue for therapeutic intervention during the transition period.
Keywords: GABA; L-type voltage-gated calcium channel (LTCC); alcohol/ethanol; central amygdala; corticotropin-releasing factor type 1 receptor (CRF1).
Copyright © 2017 the authors 0270-6474/17/374593-11$15.00/0.
Figures



References
MeSH terms
Substances
Grants and funding
- P50 AA006420/AA/NIAAA NIH HHS/United States
- U01 AA013498/AA/NIAAA NIH HHS/United States
- R37 AA017447/AA/NIAAA NIH HHS/United States
- K99 AA021802/AA/NIAAA NIH HHS/United States
- R01 AA012602/AA/NIAAA NIH HHS/United States
- R01 AA017447/AA/NIAAA NIH HHS/United States
- P60 AA006420/AA/NIAAA NIH HHS/United States
- R01 AA015566/AA/NIAAA NIH HHS/United States
- R00 AA021802/AA/NIAAA NIH HHS/United States
- R01 AA021491/AA/NIAAA NIH HHS/United States
- R01 AA022977/AA/NIAAA NIH HHS/United States
- R01 AA020608/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
