Progesterone induces neuroprotection following reperfusion-promoted mitochondrial dysfunction after focal cerebral ischemia in rats
- PMID: 28363987
- PMCID: PMC5482998
- DOI: 10.1242/dmm.025692
Progesterone induces neuroprotection following reperfusion-promoted mitochondrial dysfunction after focal cerebral ischemia in rats
Abstract
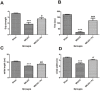
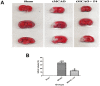
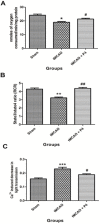
Organelle damage and increases in mitochondrial permeabilization are key events in the development of cerebral ischemic tissue injury because they cause both modifications in ATP turnover and cellular apoptosis/necrosis. Early restoration of blood flow and improvement of mitochondrial function might reverse the situation and help in recovery following an onset of stroke. Mitochondria and related bioenergetic processes can be effectively used as pharmacological targets. Progesterone (P4), one of the promising neurosteroids, has been found to be neuroprotective in various models of neurological diseases, through a number of mechanisms. This influenced us to investigate the possible role of P4 in the mitochondria-mediated neuroprotective mechanism in an ischemic stroke model of rat. In this study, we have shown the positive effect of P4 administration on behavioral deficits and mitochondrial health in an ischemic stroke injury model of transient middle cerebral artery occlusion (tMCAO). After induction of tMCAO, the rats received an initial intraperitoneal injection of P4 (8 mg/kg body weight) or vehicle at 1 h post-occlusion followed by subcutaneous injections at 6, 12 and 18 h. Behavioral assessment for functional deficits included grip strength, motor coordination and gait analysis. Findings revealed a significant improvement with P4 treatment in tMCAO animals. Staining of isolated brain slices from P4-treated rats with 2,3,5-triphenyltetrazolium chloride (TTC) showed a reduction in the infarct area in comparison to the vehicle group, indicating the presence of an increased number of viable mitochondria. P4 treatment was also able to attenuate mitochondrial reactive oxygen species (ROS) production, as well as block the mitochondrial permeability transition pore (mPTP), in the tMCAO injury model. In addition, it was also able to ameliorate the altered mitochondrial membrane potential and respiration ratio in the ischemic animals, thereby suggesting that P4 has a positive effect on mitochondrial bioenergetics. In conclusion, these results demonstrate that P4 treatment is beneficial in preserving the mitochondrial functions that are altered in cerebral ischemic injury and thus can help in defining better therapies.
Keywords: Apoptosis; Cerebral ischemia; Mitochondria; Neurobehavior; Neuroprotection; Progesterone.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





References
-
- Affonso A. C., Machado D. G., Malgarin F., Fraga D. B., Ghedim F., Zugno A., Streck E. L., Schuck P. F. and Ferreira G. C. (2013). Increased susceptibility of brain acetylcholinesterase activity to methylmalonate in young rats with renal failure. Metab. Brain Dis. 28, 493-500. 10.1007/s11011-013-9396-0 - DOI - PubMed
-
- Ashafaq M., Khan M. M., Shadab Raza S., Ahmad A., Khuwaja G., Javed H., Khan A., Islam F., Siddiqui M. S., Safhi M. M. et al. (2012a). S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutr. Res. 32, 133-143. 10.1016/j.nutres.2011.12.014 - DOI - PubMed
-
- Ashafaq M., Raza S. S., Khan M. M., Ahmad A., Javed H., Ahmad M. E., Tabassum R., Islam F., Siddiqui M. S., Safhi M. M. et al. (2012b). Catechin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia. Neurochem. Res. 37, 1747-1760. 10.1007/s11064-012-0786-1 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

