Response Heterogeneity of EGFR and HER2 Exon 20 Insertions to Covalent EGFR and HER2 Inhibitors
- PMID: 28363995
- PMCID: PMC5596996
- DOI: 10.1158/0008-5472.CAN-16-3404
Response Heterogeneity of EGFR and HER2 Exon 20 Insertions to Covalent EGFR and HER2 Inhibitors
Abstract
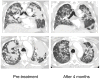
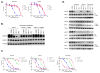
Insertion mutations in EGFR and HER2 both occur at analogous positions in exon 20. Non-small cell lung cancer (NSCLC) patients with tumors harboring these mutations seldom achieve clinical responses to dacomitinib and afatinib, two covalent quinazoline-based inhibitors of EGFR or HER2, respectively. In this study, we investigated the effects of specific EGFR and HER2 exon 20 insertion mutations from NSCLC patients that had clinically achieved a partial response after dacomitinib treatment. We identified Gly770 as a common feature among the drug-sensitive mutations. Structural modeling suggested that this mutation may facilitate inhibitor binding to EGFR. Introduction of Gly770 into two dacomitinib-resistant EGFR exon 20 insertion mutants restored sensitivity to dacomitinib. Based on these findings, we used afatinib to treat an NSCLC patient whose tumor harbored the HER2 V777_G778insGSP mutation and achieved a durable partial response. We further identified secondary mutations in EGFR (T790M or C797S) and HER2 (C805S) that mediated acquired drug resistance in drug-sensitive EGFR or HER2 exon 20 insertion models. Overall, our findings identified a subset of EGFR and HER2 exon 20 insertion mutations that are sensitive to existing covalent quinazoline-based EGFR/HER2 inhibitors, with implications for current clinical treatment and next-generation small-molecule inhibitors. Cancer Res; 77(10); 2712-21. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures


References
-
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. - PubMed
-
- Solomon BJ, Mok T. First-line crizotinib in ALK-positive lung cancer. N Engl J Med. 2015;372:782. - PubMed
-
- Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. - PubMed
-
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

