Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models
- PMID: 28364014
- PMCID: PMC5540794
- DOI: 10.1158/1078-0432.CCR-16-2955
Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models
Abstract
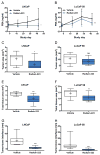
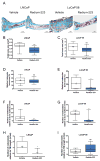
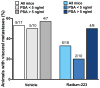
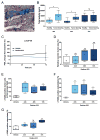
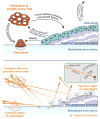
Purpose: Radium-223 dichloride (radium-223, Xofigo), a targeted alpha therapy, is currently used for the treatment of patients with castration-resistant prostate cancer (CRPC) with bone metastases. This study examines the mode-of-action and antitumor efficacy of radium-223 in two prostate cancer xenograft models.Experimental Design: Mice bearing intratibial LNCaP or LuCaP 58 tumors were randomized into groups (n = 12-17) based on lesion grade and/or serum PSA level and administered radium-223 (300 kBq/kg) or vehicle, twice at 4-week intervals. X-rays and serum samples were obtained biweekly. Soft tissue tumors were observed macroscopically at sacrifice. Tibiae were analyzed by gamma counter, micro-CT, autoradiography and histology.Results: Radium-223 inhibited tumor-induced osteoblastic bone growth and protected normal bone architecture, leading to reduced bone volume in LNCaP and abiraterone-resistant LuCaP 58 models. Furthermore, radium-223 resulted in lower PSA values and reduced total tissue and tumor areas, indicating that treatment constrains prostate cancer growth in bone. In addition, radium-223 suppressed abnormal bone metabolic activity as evidenced by decreased number of osteoblasts and osteoclasts and reduced level of the bone formation marker PINP. Mode-of-action studies revealed that radium-223 was deposited in the intratumoral bone matrix. DNA double-strand breaks were induced in cancer cells within 24 hours after radium-223 treatment, and PSA levels were significantly lower 72 hours after treatment, providing further evidence of the antitumor effects.Conclusions: Taken together, radium-223 therapy exhibits a dual targeting mode-of-action that induces tumor cell death and suppresses tumor-induced pathologic bone formation in tumor microenvironment of osseous CRPC growth in mice. Clin Cancer Res; 23(15); 4335-46. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures

References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Vignani F, Bertaglia V, Buttigliero C, Tucci M, Scagliotti GV, Di Maio M. Skeletal metastases and impact of anticancer and bone-targeted agents in patients with castration-resistant prostate cancer. Cancer Treat Rev. 2016;44:61–73. - PubMed
-
- Broder MS, Gutierrez B, Cherepanov D, Linhares Y. Burden of skeletal-related events in prostate cancer: unmet need in pain improvement. Support Care Cancer. 2015;23:237–47. - PubMed
-
- Jayasekera J, Onukwugha E, Bikov K, Mullins CD, Seal B, Hussain A. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics. 2014;32:173–91. - PubMed
-
- Fizazi K, Scher HI, Miller K, Basch E, Sternberg CN, Cella D, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

