Lateral Segregation of Photosystem I in Cyanobacterial Thylakoids
- PMID: 28364021
- PMCID: PMC5466035
- DOI: 10.1105/tpc.17.00071
Lateral Segregation of Photosystem I in Cyanobacterial Thylakoids
Erratum in
-
CORRECTION.Plant Cell. 2017 Jul;29(7):1794. doi: 10.1105/tpc.17.00535. Epub 2017 Jul 6. Plant Cell. 2017. PMID: 28687654 Free PMC article. No abstract available.
Abstract
Photosystem I (PSI) is the dominant photosystem in cyanobacteria and it plays a pivotal role in cyanobacterial metabolism. Despite its biological importance, the native organization of PSI in cyanobacterial thylakoid membranes is poorly understood. Here, we use atomic force microscopy (AFM) to show that ordered, extensive macromolecular arrays of PSI complexes are present in thylakoids from Thermosynechococcus elongatus, Synechococcus sp PCC 7002, and Synechocystis sp PCC 6803. Hyperspectral confocal fluorescence microscopy and three-dimensional structured illumination microscopy of Synechocystis sp PCC 6803 cells visualize PSI domains within the context of the complete thylakoid system. Crystallographic and AFM data were used to build a structural model of a membrane landscape comprising 96 PSI trimers and 27,648 chlorophyll a molecules. Rather than facilitating intertrimer energy transfer, the close associations between PSI primarily maximize packing efficiency; short-range interactions with Complex I and cytochrome b6f are excluded from these regions of the membrane, so PSI turnover is sustained by long-distance diffusion of the electron donors at the membrane surface. Elsewhere, PSI-photosystem II contact zones provide sites for docking phycobilisomes and the formation of megacomplexes. PSI-enriched domains in cyanobacteria might foreshadow the partitioning of PSI into stromal lamellae in plants, similarly sustained by long-distance diffusion of electron carriers.
© 2017 American Society of Plant Biologists. All rights reserved.
Figures

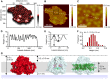


 of excitation at RCI as a function of initial site across the PSI monomers M = I, II, III, II', III' as indicated in (B). Chlorophyll number m corresponds to the order given in PDB:1JB0. As suggested by the connections in (A), excitation sharing within the monomers of the same trimer is more prominent than across trimers.
of excitation at RCI as a function of initial site across the PSI monomers M = I, II, III, II', III' as indicated in (B). Chlorophyll number m corresponds to the order given in PDB:1JB0. As suggested by the connections in (A), excitation sharing within the monomers of the same trimer is more prominent than across trimers.

Similar articles
-
Dissecting the Native Architecture and Dynamics of Cyanobacterial Photosynthetic Machinery.Mol Plant. 2017 Nov 6;10(11):1434-1448. doi: 10.1016/j.molp.2017.09.019. Epub 2017 Oct 7. Mol Plant. 2017. PMID: 29017828 Free PMC article.
-
Pigment-protein complexes are organized into stable microdomains in cyanobacterial thylakoids.Biochim Biophys Acta Bioenerg. 2019 Dec 1;1860(12):148053. doi: 10.1016/j.bbabio.2019.07.008. Epub 2019 Jul 22. Biochim Biophys Acta Bioenerg. 2019. PMID: 31344362
-
Structural variability, coordination and adaptation of a native photosynthetic machinery.Nat Plants. 2020 Jul;6(7):869-882. doi: 10.1038/s41477-020-0694-3. Epub 2020 Jul 13. Nat Plants. 2020. PMID: 32665651
-
Energy transfer and distribution in photosystem super/megacomplexes of plants.Curr Opin Biotechnol. 2018 Dec;54:50-56. doi: 10.1016/j.copbio.2018.01.001. Epub 2018 Feb 20. Curr Opin Biotechnol. 2018. PMID: 29475052 Review.
-
Long-wavelength chlorophylls in photosystem I of cyanobacteria: origin, localization, and functions.Biochemistry (Mosc). 2014 Mar;79(3):213-20. doi: 10.1134/S0006297914030067. Biochemistry (Mosc). 2014. PMID: 24821447 Review.
Cited by
-
Lighting the way: Compelling open questions in photosynthesis research.Plant Cell. 2024 Oct 3;36(10):3914-3943. doi: 10.1093/plcell/koae203. Plant Cell. 2024. PMID: 39038210 Free PMC article.
-
Advances in the Understanding of the Lifecycle of Photosystem II.Microorganisms. 2022 Apr 19;10(5):836. doi: 10.3390/microorganisms10050836. Microorganisms. 2022. PMID: 35630282 Free PMC article. Review.
-
Functional consequences of modification of the photosystem I/photosystem II ratio in the cyanobacterium Synechocystis sp. PCC 6803.J Bacteriol. 2024 May 23;206(5):e0045423. doi: 10.1128/jb.00454-23. Epub 2024 May 2. J Bacteriol. 2024. PMID: 38695523 Free PMC article.
-
State transitions and photosystems spatially resolved in individual cells of the cyanobacterium Synechococcus elongatus.Plant Physiol. 2021 May 27;186(1):569-580. doi: 10.1093/plphys/kiab063. Plant Physiol. 2021. PMID: 33576804 Free PMC article.
-
Physiological and evolutionary implications of tetrameric photosystem I in cyanobacteria.Nat Plants. 2019 Dec;5(12):1309-1319. doi: 10.1038/s41477-019-0566-x. Epub 2019 Dec 9. Nat Plants. 2019. PMID: 31819227
References
-
- Agarwal R., Matros A., Melzer M., Mock H.-P., Sainis J.K. (2010). Heterogeneity in thylakoid membrane proteome of Synechocystis 6803. J. Proteomics 73: 976–991. - PubMed
-
- Biggins J., Bruce D. (1989). Regulation of excitation energy transfer in organisms containing phycobilins. Photosynth. Res. 20: 1–34. - PubMed
-
- Biggins J., Campbell C.L., Bruce D. (1984). Mechanism of the light state transition in photosynthesis. II. Analysis of phosphorylated polypeptides in the red alga, Porphyridium cruentum. Biochim. Biophys. Acta 767: 138–144.
-
- Boussac A., Rappaport F., Carrier P., Verbavatz J.-M., Gobin R., Kirilovsky D., Rutherford A.W., Sugiura M. (2004). Biosynthetic Ca2+/Sr2+ exchange in the photosystem II oxygen-evolving enzyme of Thermosynechococcus elongatus. J. Biol. Chem. 279: 22809–22819. - PubMed
-
- Bryant D.A., ed (2006). The Molecular Biology of Cyanobacteria, Vol. 1. (Dordrecht, The Netherlands: Springer Science & Business Media).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

