Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells
- PMID: 28364041
- PMCID: PMC5437243
- DOI: 10.1074/jbc.M117.783431
Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells
Abstract
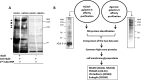
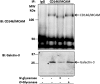
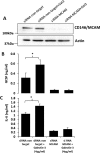
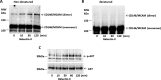
The galactoside-binding protein galectin-3 is increasingly recognized as an important player in cancer development, progression, and metastasis via its interactions with various galactoside-terminated glycans. We have shown previously that circulating galectin-3, which is increased up to 30-fold in cancer patients, promotes blood-borne metastasis in an animal cancer model. This effect is partly attributable to the interaction of galectin-3 with unknown receptor(s) on vascular endothelial cells and causes endothelial secretion of several metastasis-promoting cytokines. Here we sought to identify the galectin-3-binding molecule(s) on the endothelial cell surface responsible for the galectin-3-mediated cytokine secretion. Using two different galectin-3 affinity purification processes, we extracted four cell membrane glycoproteins, CD146/melanoma cell adhesion molecule (MCAM)/MUC18, CD31/platelet endothelial cell adhesion molecule-1 (PECAM-1), CD144/VE-cadherin, and CD106/Endoglin, from vascular endothelial cells. CD146 was the major galectin-3-binding ligand and strongly co-localized with galectin-3 on endothelial cell surfaces treated with exogenous galectin-3. Moreover, galectin-3 bound to N-linked glycans on CD146 and induced CD146 dimerization and subsequent activation of AKT signaling. siRNA-mediated suppression of CD146 expression completely abolished the galectin-3-induced secretion of IL-6 and G-CSF cytokines from the endothelial cells. Thus, CD146/MCAM is the functional galectin-3-binding ligand on endothelial cell surfaces responsible for galectin-3-induced secretion of metastasis-promoting cytokines. We conclude that CD146/MCAM interactions with circulating galectin-3 may have an important influence on cancer progression and metastasis.
Keywords: CD146; IL-6; MCAM; carbohydrate-binding protein; cytokine; endothelium; galectin; galectin-3.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Galectin-3 Is a Natural Binding Ligand of MCAM (CD146, MUC18) in Melanoma Cells and Their Interaction Promotes Melanoma Progression.Biomolecules. 2022 Oct 10;12(10):1451. doi: 10.3390/biom12101451. Biomolecules. 2022. PMID: 36291660 Free PMC article.
-
Increased circulation of galectin-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium.Clin Cancer Res. 2013 Apr 1;19(7):1693-704. doi: 10.1158/1078-0432.CCR-12-2940. Epub 2013 Feb 11. Clin Cancer Res. 2013. PMID: 23401226 Free PMC article.
-
MUC18/MCAM (CD146), an activation antigen of human T lymphocytes.J Immunol. 1997 Mar 1;158(5):2107-15. J Immunol. 1997. PMID: 9036955
-
CD146, from a melanoma cell adhesion molecule to a signaling receptor.Signal Transduct Target Ther. 2020 Aug 11;5(1):148. doi: 10.1038/s41392-020-00259-8. Signal Transduct Target Ther. 2020. PMID: 32782280 Free PMC article. Review.
-
Melanoma Cell Adhesion Molecule (CD 146) in Endometrial Physiology and Disorder.Adv Exp Med Biol. 2025;1474:131-148. doi: 10.1007/5584_2024_826. Adv Exp Med Biol. 2025. PMID: 39400880 Review.
Cited by
-
Endoglin Protein Interactome Profiling Identifies TRIM21 and Galectin-3 as New Binding Partners.Cells. 2019 Sep 13;8(9):1082. doi: 10.3390/cells8091082. Cells. 2019. PMID: 31540324 Free PMC article.
-
Genomic, proteomic, and immunologic associations with a durable complete remission of measurable metastatic melanoma induced by a patient-specific dendritic cell vaccine.Hum Vaccin Immunother. 2020 Apr 2;16(4):742-755. doi: 10.1080/21645515.2019.1680239. Epub 2019 Nov 15. Hum Vaccin Immunother. 2020. PMID: 31625825 Free PMC article.
-
Appearance of peanut agglutinin in the blood circulation after peanut ingestion promotes endothelial secretion of metastasis-promoting cytokines.Carcinogenesis. 2021 Aug 19;42(8):1079-1088. doi: 10.1093/carcin/bgab059. Carcinogenesis. 2021. PMID: 34223877 Free PMC article.
-
The Possible Effects of Galectin-3 on Mechanisms of Renal and Hepatocellular Injury Induced by Intravascular Hemolysis.Int J Mol Sci. 2024 Jul 25;25(15):8129. doi: 10.3390/ijms25158129. Int J Mol Sci. 2024. PMID: 39125698 Free PMC article. Review.
-
CD146 is a Novel ANGPTL2 Receptor that Promotes Obesity by Manipulating Lipid Metabolism and Energy Expenditure.Adv Sci (Weinh). 2021 Jan 27;8(6):2004032. doi: 10.1002/advs.202004032. eCollection 2021 Mar. Adv Sci (Weinh). 2021. PMID: 33747748 Free PMC article.
References
-
- Liu F. T., and Rabinovich G. A. (2005) Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41 - PubMed
-
- Newlaczyl A. U., and Yu L. G. (2011) Galectin-3: a jack-of-all-trades in cancer. Cancer Lett. 313, 123–128 - PubMed
-
- Barrow H., Guo X., Wandall H. H., Pedersen J. W., Fu B., Zhao Q., Chen C., Rhodes J. M., and Yu L. G. (2011) Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin. Cancer Res. 17, 7035–7046 - PubMed
-
- Iurisci I., Tinari N., Natoli C., Angelucci D., Cianchetti E., and Iacobelli S. (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin. Cancer Res. 6, 1389–1393 - PubMed
-
- Sakaki M., Oka N., Nakanishi R., Yamaguchi K., Fukumori T., and Kanayama H. O. (2008) Serum level of galectin-3 in human bladder cancer. J. Med. Invest. 55, 127–132 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

