Kinetic-based trapping by intervening sequence variants of the active sites of protein-disulfide isomerase identifies platelet protein substrates
- PMID: 28364042
- PMCID: PMC5454092
- DOI: 10.1074/jbc.M116.771832
Kinetic-based trapping by intervening sequence variants of the active sites of protein-disulfide isomerase identifies platelet protein substrates
Abstract
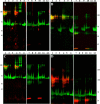
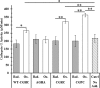
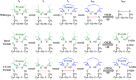
Thiol isomerases such as protein-disulfide isomerase (PDI) direct disulfide rearrangements required for proper folding of nascent proteins synthesized in the endoplasmic reticulum. Identifying PDI substrates is challenging because PDI catalyzes conformational changes that cannot be easily monitored (e.g. compared with proteolytic cleavage or amino acid phosphorylation); PDI has multiple substrates; and it can catalyze either oxidation, reduction, or isomerization of substrates. Kinetic-based substrate trapping wherein the active site motif CGHC is modified to CGHA to stabilize a PDI-substrate intermediate is effective in identifying some substrates. A limitation of this approach, however, is that it captures only substrates that are reduced by PDI, whereas many substrates are oxidized by PDI. By manipulating the highly conserved -GH- residues in the CGHC active site of PDI, we created PDI variants with a slowed reaction rate toward substrates. The prolonged intermediate state allowed us to identify protein substrates that have biased affinities for either oxidation or reduction by PDI. Because extracellular PDI is critical for thrombus formation but its extracellular substrates are not known, we evaluated the ability of these bidirectional trapping PDI variants to trap proteins released from platelets and on the platelet surface. Trapped proteins were identified by mass spectroscopy. Of the trapped substrate proteins identified by mass spectroscopy, five proteins, cathepsin G, glutaredoxin-1, thioredoxin, GP1b, and fibrinogen, showed a bias for oxidation, whereas annexin V, heparanase, ERp57, kallekrein-14, serpin B6, tetranectin, and collagen VI showed a bias for reduction. These bidirectional trapping variants will enable more comprehensive identification of thiol isomerase substrates and better elucidation of their cellular functions.
Keywords: disulfide; oxidase; platelet; protein disulfide isomerase; protein-protein interaction; reductase.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
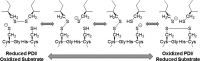
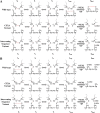
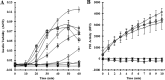
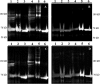
 , CGDC-PDI. RFU, relative fluorescence units.
, CGDC-PDI. RFU, relative fluorescence units.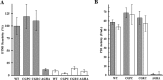


Similar articles
-
Identification of PDI Substrates by Mechanism-Based Kinetic Trapping.Methods Mol Biol. 2019;1967:165-182. doi: 10.1007/978-1-4939-9187-7_10. Methods Mol Biol. 2019. PMID: 31069770
-
Novel antiplatelet role for a protein disulfide isomerase-targeted peptide: evidence of covalent binding to the C-terminal CGHC redox motif.J Thromb Haemost. 2017 Apr;15(4):774-784. doi: 10.1111/jth.13633. Epub 2017 Feb 28. J Thromb Haemost. 2017. PMID: 28109047
-
Regulatory role of thiol isomerases in thrombus formation.Expert Rev Hematol. 2018 May;11(5):437-448. doi: 10.1080/17474086.2018.1452612. Epub 2018 Mar 28. Expert Rev Hematol. 2018. PMID: 29542339 Free PMC article. Review.
-
Catalysis of thiol/disulfide exchange. Glutaredoxin 1 and protein-disulfide isomerase use different mechanisms to enhance oxidase and reductase activities.J Biol Chem. 2005 Jun 3;280(22):21099-106. doi: 10.1074/jbc.M411476200. Epub 2005 Apr 6. J Biol Chem. 2005. PMID: 15814611
-
Extracellular Thiol Isomerases and Their Role in Thrombus Formation.Antioxid Redox Signal. 2016 Jan 1;24(1):1-15. doi: 10.1089/ars.2015.6530. Epub 2015 Nov 18. Antioxid Redox Signal. 2016. PMID: 26467859 Free PMC article. Review.
Cited by
-
Protein disulfide isomerase plasma levels in healthy humans reveal proteomic signatures involved in contrasting endothelial phenotypes.Redox Biol. 2019 Apr;22:101142. doi: 10.1016/j.redox.2019.101142. Epub 2019 Feb 19. Redox Biol. 2019. PMID: 30870787 Free PMC article.
-
The intersection of protein disulfide isomerase and cancer associated thrombosis.Thromb Res. 2018 Apr;164 Suppl 1(Suppl 1):S130-S135. doi: 10.1016/j.thromres.2018.01.005. Thromb Res. 2018. PMID: 29703471 Free PMC article. Review.
-
In Vivo Structure-Function Analysis and Redox Interactomes of Leishmania tarentolae Erv.Microbiol Spectr. 2021 Oct 31;9(2):e0080921. doi: 10.1128/Spectrum.00809-21. Epub 2021 Sep 29. Microbiol Spectr. 2021. PMID: 34585988 Free PMC article.
-
Recent advances in vascular thiol isomerases: insights into structures, functions in thrombosis and antithrombotic inhibitor development.Thromb J. 2025 Feb 17;23(1):16. doi: 10.1186/s12959-025-00699-8. Thromb J. 2025. PMID: 39962537 Free PMC article. Review.
-
Protein disulfide isomerase in cardiovascular disease.Exp Mol Med. 2020 Mar;52(3):390-399. doi: 10.1038/s12276-020-0401-5. Epub 2020 Mar 18. Exp Mol Med. 2020. PMID: 32203104 Free PMC article. Review.
References
-
- Appenzeller-Herzog C., and Ellgaard L. (2008) The human PDI family: versatility packed into a single fold. Biochim. Biophys. Acta 1783, 535–548 - PubMed
-
- Lyles M. M., and Gilbert H. F. (1991) Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: dependence of the rate on the composition of the redox buffer. Biochemistry 30, 613–619 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

