Spatial distribution and molecular dynamics of dystrophin glycoprotein components at the neuromuscular junction in vivo
- PMID: 28364093
- PMCID: PMC5450190
- DOI: 10.1242/jcs.198358
Spatial distribution and molecular dynamics of dystrophin glycoprotein components at the neuromuscular junction in vivo
Abstract
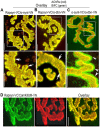
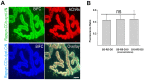
A bimolecular fluorescence complementation (BiFC) approach was used to study the molecular interactions between different components of the postsynaptic protein complex at the neuromuscular junction of living mice. We show that rapsyn forms complex with both α-dystrobrevin and α-syntrophin at the crests of junctional folds. The linkage of rapsyn to α-syntrophin and/or α-dystrobrevin is mediated by utrophin, a protein localized at acetylcholine receptor (AChR)-rich domains. In mice deficient in α-syntrophin, in which utrophin is no longer present at the synapse, rapsyn interaction with α-dystrobrevin was completely abolished. This interaction was completely restored when either utrophin or α-syntrophin was introduced into muscles deficient in α-syntrophin. However, in neuromuscular junctions deficient in α-dystrobrevin, in which utrophin is retained, complex formation between rapsyn and α-syntrophin was unaffected. Using fluorescence recovery after photobleaching, we found that α-syntrophin turnover is 5-7 times faster than that of AChRs, and loss of α-dystrobrevin has no effect on rapsyn and α-syntrophin half-life, whereas the half-life of AChR was significantly altered. Altogether, these results provide new insights into the spatial distribution of dystrophin glycoprotein components and their dynamics in living mice.
Keywords: AChR; DGC; Dystrophin glycoprotein complex; NMJ; Neuromuscular junction.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





Similar articles
-
The alpha-syntrophin PH and PDZ domains scaffold acetylcholine receptors, utrophin, and neuronal nitric oxide synthase at the neuromuscular junction.J Neurosci. 2010 Aug 18;30(33):11004-10. doi: 10.1523/JNEUROSCI.1930-10.2010. J Neurosci. 2010. PMID: 20720107 Free PMC article.
-
The postsynaptic submembrane machinery at the neuromuscular junction: requirement for rapsyn and the utrophin/dystrophin-associated complex.J Neurocytol. 2003 Jun-Sep;32(5-8):709-26. doi: 10.1023/B:NEUR.0000020619.24681.2b. J Neurocytol. 2003. PMID: 15034263 Review.
-
Defects in neuromuscular junction structure in dystrophic muscle are corrected by expression of a NOS transgene in dystrophin-deficient muscles, but not in muscles lacking alpha- and beta1-syntrophins.Hum Mol Genet. 2004 Sep 1;13(17):1873-84. doi: 10.1093/hmg/ddh204. Epub 2004 Jul 6. Hum Mol Genet. 2004. PMID: 15238508
-
Src-family kinases stabilize the neuromuscular synapse in vivo via protein interactions, phosphorylation, and cytoskeletal linkage of acetylcholine receptors.J Neurosci. 2005 Nov 9;25(45):10479-93. doi: 10.1523/JNEUROSCI.2103-05.2005. J Neurosci. 2005. PMID: 16280586 Free PMC article.
-
Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses.Mol Neurobiol. 2002 Feb;25(1):79-112. doi: 10.1385/MN:25:1:079. Mol Neurobiol. 2002. PMID: 11890459 Review.
Cited by
-
Defects of full-length dystrophin trigger retinal neuron damage and synapse alterations by disrupting functional autophagy.Cell Mol Life Sci. 2021 Feb;78(4):1615-1636. doi: 10.1007/s00018-020-03598-5. Epub 2020 Aug 4. Cell Mol Life Sci. 2021. PMID: 32749504 Free PMC article.
-
The role of Rapsyn in neuromuscular junction and congenital myasthenic syndrome.Biomol Biomed. 2023 Sep 4;23(5):772-784. doi: 10.17305/bb.2022.8641. Biomol Biomed. 2023. PMID: 36815443 Free PMC article. Review.
-
Distinct roles of the dystrophin-glycoprotein complex: α-dystrobrevin and α-syntrophin in the maintenance of the postsynaptic apparatus of the neuromuscular synapse.Hum Mol Genet. 2022 Jul 21;31(14):2370-2385. doi: 10.1093/hmg/ddac041. Hum Mol Genet. 2022. PMID: 35157076 Free PMC article.
-
Arhgef5 Binds α-Dystrobrevin 1 and Regulates Neuromuscular Junction Integrity.Front Mol Neurosci. 2020 Jun 10;13:104. doi: 10.3389/fnmol.2020.00104. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32587503 Free PMC article.
-
Utrophin up-regulation by artificial transcription factors induces muscle rescue and impacts the neuromuscular junction in mdx mice.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt A):1172-1182. doi: 10.1016/j.bbadis.2018.01.030. Epub 2018 Jan 31. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29408646 Free PMC article.
References
-
- Ahn A. H., Freener C. A., Gussoni E., Yoshida M., Ozawa E. and Kunkel L. M. (1996). The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal locations, and each bind to dystrophin and its relatives. J. Biol. Chem. 271, 2724-2730. 10.1074/jbc.271.5.2724 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

