Spatial distribution and molecular dynamics of dystrophin glycoprotein components at the neuromuscular junction in vivo
- PMID: 28364093
- PMCID: PMC5450190
- DOI: 10.1242/jcs.198358
Spatial distribution and molecular dynamics of dystrophin glycoprotein components at the neuromuscular junction in vivo
Abstract
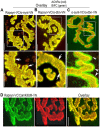
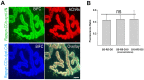
A bimolecular fluorescence complementation (BiFC) approach was used to study the molecular interactions between different components of the postsynaptic protein complex at the neuromuscular junction of living mice. We show that rapsyn forms complex with both α-dystrobrevin and α-syntrophin at the crests of junctional folds. The linkage of rapsyn to α-syntrophin and/or α-dystrobrevin is mediated by utrophin, a protein localized at acetylcholine receptor (AChR)-rich domains. In mice deficient in α-syntrophin, in which utrophin is no longer present at the synapse, rapsyn interaction with α-dystrobrevin was completely abolished. This interaction was completely restored when either utrophin or α-syntrophin was introduced into muscles deficient in α-syntrophin. However, in neuromuscular junctions deficient in α-dystrobrevin, in which utrophin is retained, complex formation between rapsyn and α-syntrophin was unaffected. Using fluorescence recovery after photobleaching, we found that α-syntrophin turnover is 5-7 times faster than that of AChRs, and loss of α-dystrobrevin has no effect on rapsyn and α-syntrophin half-life, whereas the half-life of AChR was significantly altered. Altogether, these results provide new insights into the spatial distribution of dystrophin glycoprotein components and their dynamics in living mice.
Keywords: AChR; DGC; Dystrophin glycoprotein complex; NMJ; Neuromuscular junction.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





References
-
- Ahn A. H., Freener C. A., Gussoni E., Yoshida M., Ozawa E. and Kunkel L. M. (1996). The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal locations, and each bind to dystrophin and its relatives. J. Biol. Chem. 271, 2724-2730. 10.1074/jbc.271.5.2724 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

