Using Oscillating Sounds to Manipulate Sleep Spindles
- PMID: 28364415
- PMCID: PMC6084765
- DOI: 10.1093/sleep/zsw068
Using Oscillating Sounds to Manipulate Sleep Spindles
Abstract
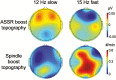
Introduction: EEG oscillations known as sleep spindles have been linked with various aspects of cognition, but the specific functions they signal remain controversial. Two types of EEG sleep spindles have been distinguished: slow spindles at 11-13.5 Hz and fast spindles at 13.5-16 Hz. Slow spindles exhibit a frontal scalp topography, whereas fast spindles exhibit a posterior scalp topography and have been preferentially linked with memory consolidation during sleep. To advance understanding beyond that provided from correlative studies of spindles, we aimed to develop a new method to systematically manipulate spindles.
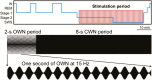
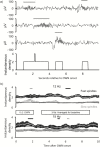
Aims and methods: We presented repeating bursts of oscillating white noise to people during a 90-min afternoon nap. During stage 2 and slow-wave sleep, oscillations were embedded within contiguous 10-s stimulation intervals, each comprising 2 s of white noise amplitude modulated at 12 Hz (targeting slow spindles), 15 Hz (targeting fast spindles), or 50 Hz followed by 8 s of constant white noise.
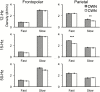
Results: During oscillating stimulation compared to constant stimulation, parietal EEG recordings showed more slow spindles in the 12-Hz condition, more fast spindles in the 15-Hz condition, and no change in the 50-Hz control condition. These effects were topographically selective, and were absent in frontopolar EEG recordings, where slow spindle density was highest. Spindles during stimulation were similar to spontaneous spindles in standard physiological features, including duration and scalp distribution.
Conclusions: These results define a new method to selectively and noninvasively manipulate spindles through acoustic resonance, while also providing new evidence for functional distinctions between the 2 types of EEG spindles.
Keywords: memory consolidation.; oscillations; sleep spindles.
© Sleep Research Society 2016. Published by Oxford University Press on behalf of the Sleep Research Society. All rights reserved. For permissions, please e-mail journals.permissions@oup.com.
Figures
References
-
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003; 7(5): 423–440. - PubMed
-
- Astori S, Wimmer RD, Lüthi A. Manipulating sleep spindles–expanding views on sleep, memory, and disease. Trends Neurosci. 2013; 36(12): 738–748. - PubMed
-
- Gibbs F, Gibbs E. An Atlas of Electroencephalography. Vol 1 Cambridge: Addison-Wesley Press; 1950.
-
- Anderer P, Klösch G, Gruber G, et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001; 103(3): 581–592. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

