Glutamate Is a Wake-Active Neurotransmitter in Drosophila melanogaster
- PMID: 28364503
- PMCID: PMC6084761
- DOI: 10.1093/sleep/zsw046
Glutamate Is a Wake-Active Neurotransmitter in Drosophila melanogaster
Abstract
Introduction: In mammals, there is evidence that glutamate has a role as a wake-active neurotransmitter. So using video-based analysis of Drosophila behavior, we undertook a study to examine if glutamate, which has been previously shown to have an excitatory role in neuromuscular junctions in Drosophila, may have a conserved wake-active role in the adult brain.
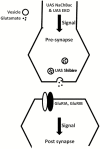
Aims and methods: Using 6- to 9-day-old female flies, we examined the effect of perturbations of the glutamatergic signaling on total wakefulness and wake bout architecture. We increased and decreased neuronal activity of glutamatergic neurons in the brains of adult flies using Upstream Activating Sequence (UAS) NaChBac and UAS EKO, respectively. We blocked neurotransmission from glutamatergic neurons in adult flies using the UAS-driven temperature-sensitive dynamin mutation shibirets. We examined the behavior of flies with loss of function mutations of individual subunits of brain-specific ionotropic glutamate receptors.
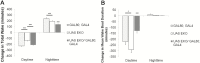
Results: Increasing the activity of glutamatergic neurons in the adult brain led to a significant increase in wakefulness compared to the control groups both in the daytime and nighttime and decreasing the activity of these same neurons reduced wakefulness in the nighttime. Blocking neurotransmitter release in glutamatergic neurons significantly reduced wake in the nighttime. The ionotropic receptor mutants had significantly less wake in the nighttime than their respective genetic background controls.
Conclusion: The results show the following: glutamate is indeed a wake-active neurotransmitter in Drosophila; there is a major time of day effect associated with loss of glutamatergic neurotransmission; and it is a major wake-active neurotransmitter in the nighttime.
Keywords: Drosophila melanogaster; sleep duration.; synaptic plasticity; wake bout.
© Sleep Research Society 2017. Published by Oxford University Press on behalf of the Sleep Research Society. All rights reserved. For permissions, please e-mail journals.permissions@oup.com.
Figures




Similar articles
-
Dopamine Signaling in Wake-Promoting Clock Neurons Is Not Required for the Normal Regulation of Sleep in Drosophila.J Neurosci. 2020 Dec 9;40(50):9617-9633. doi: 10.1523/JNEUROSCI.1488-20.2020. Epub 2020 Nov 10. J Neurosci. 2020. PMID: 33172977 Free PMC article.
-
Neurotransmitter levels and synaptic strength at the Drosophila larval neuromuscular junction are not altered by mutation in the sluggish-A gene, which encodes proline oxidase and affects adult locomotion.J Neurogenet. 2000 Sep;14(3):165-92. doi: 10.3109/01677060009083481. J Neurogenet. 2000. PMID: 10992167
-
[The roles of glutamate in sleep and wakefulness].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013 Sep;42(5):583-90. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013. PMID: 24167143 Review. Chinese.
-
Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4785-90. doi: 10.1073/pnas.1419603112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825756 Free PMC article.
-
[Neurochemical mechanisms of sleep regulation].Glas Srp Akad Nauka Med. 2009;(50):97-109. Glas Srp Akad Nauka Med. 2009. PMID: 20666118 Review. Serbian.
Cited by
-
Clock gene-dependent glutamate dynamics in the bean bug brain regulate photoperiodic reproduction.PLoS Biol. 2022 Sep 6;20(9):e3001734. doi: 10.1371/journal.pbio.3001734. eCollection 2022 Sep. PLoS Biol. 2022. PMID: 36067166 Free PMC article.
-
Microdosing ketamine in Drosophila does not block serotonin reuptake, but causes complex behavioral changes mediated by glutamate and serotonin receptors.J Neurochem. 2024 Jun;168(6):1097-1112. doi: 10.1111/jnc.16070. Epub 2024 Feb 7. J Neurochem. 2024. PMID: 38323657 Free PMC article.
-
Cerebral Metabolic Changes During Sleep.Curr Neurol Neurosci Rep. 2018 Jul 16;18(9):57. doi: 10.1007/s11910-018-0868-9. Curr Neurol Neurosci Rep. 2018. PMID: 30014344 Free PMC article. Review.
-
The Glutamate-gated Chloride Channel Facilitates Sleep by Enhancing the Excitability of Two Pairs of Neurons in the Ventral Nerve Cord of Drosophila.Neurosci Bull. 2025 Apr 30. doi: 10.1007/s12264-025-01397-1. Online ahead of print. Neurosci Bull. 2025. PMID: 40304877
-
Better Sleep at Night: How Light Influences Sleep in Drosophila.Front Physiol. 2020 Sep 4;11:997. doi: 10.3389/fphys.2020.00997. eCollection 2020. Front Physiol. 2020. PMID: 33013437 Free PMC article. Review.
References
-
- Schneider EH, Neumann D, Seifert R. Modulation of behavior by the histaminergic system: lessons from H(1)R-and H(2)R-deficient mice. Neurosci Biobehav Rev. 2014; 42: 252–266. - PubMed
-
- Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011; 15(4): 269–281. - PubMed
-
- Platt B, Riedel G. The cholinergic system, EEG and sleep. Behav Brain Res. 2011; 221(2): 499–504. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

