A Cell-Surface Membrane Protein Signature for Glioblastoma
- PMID: 28365151
- PMCID: PMC5512565
- DOI: 10.1016/j.cels.2017.03.004
A Cell-Surface Membrane Protein Signature for Glioblastoma
Abstract
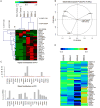
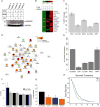
We present a systems strategy that facilitated the development of a molecular signature for glioblastoma (GBM), composed of 33 cell-surface transmembrane proteins. This molecular signature, GBMSig, was developed through the integration of cell-surface proteomics and transcriptomics from patient tumors in the REMBRANDT (n = 228) and TCGA datasets (n = 547) and can separate GBM patients from control individuals with a Matthew's correlation coefficient value of 0.87 in a lock-down test. Functionally, 17/33 GBMSig proteins are associated with transforming growth factor β signaling pathways, including CD47, SLC16A1, HMOX1, and MRC2. Knockdown of these genes impaired GBM invasion, reflecting their role in disease-perturbed changes in GBM. ELISA assays for a subset of GBMSig (CD44, VCAM1, HMOX1, and BIGH3) on 84 plasma specimens from multiple clinical sites revealed a high degree of separation of GBM patients from healthy control individuals (area under the curve is 0.98 in receiver operating characteristic). In addition, a classifier based on these four proteins differentiated the blood of pre- and post-tumor resections, demonstrating potential clinical value as biomarkers.
Keywords: GBM; GBMSig; cell-surface proteins; invasion.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Aigner L, Bogdahn U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell and tissue research. 2008;331:225–241. - PubMed
-
- Audigier Y, Picault FX, Chaves-Almagro C, Masri B. G Protein-Coupled Receptors in cancer: biochemical interactions and drug design. Progress in molecular biology and translational science. 2013;115:143–173. - PubMed
-
- Bonnefoi H, Potti A, Delorenzi M, Mauriac L, Campone M, Tubiana-Hulin M, Petit T, Rouanet P, Jassem J, Blot E, et al. Validation of gene signatures that predict the response of breast cancer to neoadjuvant chemotherapy: a substudy of the EORTC 10994/BIG 00-01 clinical trial. The Lancet Oncology. 2007;8:1071–1078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

