Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage
- PMID: 28365213
- PMCID: PMC5540140
- DOI: 10.1016/j.nbd.2017.03.016
Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage
Abstract
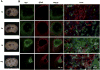
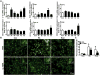
Microglia/macrophages (MMΦ) are highly plastic phagocytes that can promote both injury and repair in diseased brain through the distinct function of classically activated and alternatively activated subsets. The role of MMΦ polarization in intracerebral hemorrhage (ICH) is unknown. Herein, we comprehensively characterized MMΦ dynamics after ICH in mice and evaluated the relevance of MMΦ polarity to hematoma resolution. MMΦ accumulated within the hematoma territory until at least 14days after ICH induction. Microglia rapidly reacted to the hemorrhagic insult as early as 1-1.5h after ICH and specifically presented a "protective" alternatively activated phenotype. Substantial numbers of activated microglia and newly recruited monocytes also assumed an early alternatively activated phenotype, but the phenotype gradually shifted to a mixed spectrum over time. Ultimately, markers of MMΦ classic activation dominated at the chronic stage of ICH. We enhanced MMΦ alternative activation by administering intraperitoneal injections of rosiglitazone, and subsequently observed elevations in CD206 expression on brain-isolated CD11b+ cells and increases in IL-10 levels in serum and perihematomal tissue. Enhancement of MMΦ alternative activation correlated with hematoma volume reduction and improvement in neurologic deficits. Intraventricular injection of alternative activation signature cytokine IL-10 accelerated hematoma resolution, whereas microglial phagocytic ability was abolished by IL-10 receptor neutralization. Our results suggest that MMΦ respond dynamically to brain hemorrhage by exhibiting diverse phenotypic changes at different stages of ICH. Alternative activation-skewed MMΦ aid in hematoma resolution, and IL-10 signaling might contribute to regulation of MMΦ phagocytosis and hematoma clearance in ICH.
Keywords: Hematoma resolution; Interleukin-10; Intracerebral hemorrhage; Microglia/macrophage polarization; Phagocytosis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









References
-
- Ajmone-Cat MA, et al. Microglial polarization and plasticity: evidence from organotypic hippocampal slice cultures. Glia. 2013;61:1698–711. - PubMed
-
- Allahtavakoli M, et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, reduces infarction volume and neurological deficits in an embolic model of stroke. Clin Exp Pharmacol Physiol. 2006;33:1052–8. - PubMed
-
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–79. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

