Long-term blood glucose monitoring with implanted telemetry device in conscious and stress-free cynomolgus monkeys
- PMID: 28365864
- PMCID: PMC5559582
- DOI: 10.1007/s40618-017-0651-9
Long-term blood glucose monitoring with implanted telemetry device in conscious and stress-free cynomolgus monkeys
Abstract
Aims: Continuous blood glucose monitoring, especially long-term and remote, in diabetic patients or research is very challenging. Nonhuman primate (NHP) is an excellent model for metabolic research, because NHPs can naturally develop Type 2 diabetes mellitus (T2DM) similarly to humans. This study was to investigate blood glucose changes in conscious, moving-free cynomolgus monkeys (Macaca fascicularis) during circadian, meal, stress and drug exposure.
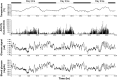
Materials and methods: Blood glucose, body temperature and physical activities were continuously and simultaneously recorded by implanted HD-XG telemetry device for up to 10 weeks.
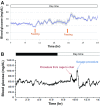
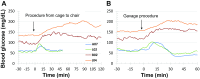
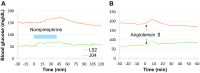
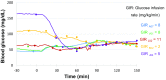
Results and discussion: Blood glucose circadian changes in normoglycemic monkeys significantly differed from that in diabetic animals. Postprandial glucose increase was more obvious after afternoon feeding. Moving a monkey from its housing cage to monkey chair increased blood glucose by 30% in both normoglycemic and diabetic monkeys. Such increase in blood glucose declined to the pre-procedure level in 30 min in normoglycemic animals and >2 h in diabetic monkeys. Oral gavage procedure alone caused hyperglycemia in both normoglycemic and diabetic monkeys. Intravenous injection with the stress hormones, angiotensin II (2 μg/kg) or norepinephrine (0.4 μg/kg), also increased blood glucose level by 30%. The glucose levels measured by the telemetry system correlated significantly well with glucometer readings during glucose tolerance tests (ivGTT or oGTT), insulin tolerance test (ITT), graded glucose infusion (GGI) and clamp.
Conclusion: Our data demonstrate that the real-time telemetry method is reliable for monitoring blood glucose remotely and continuously in conscious, stress-free, and moving-free NHPs with the advantages highly valuable to diabetes research and drug discovery.
Keywords: Continuous glucose monitoring; Diabetes; Glucose circadian; Implantable telemetry device; Nonhuman primate.
Conflict of interest statement
Conflict of interest
All of the authors are employee of Crown Bioscience Inc., except R Lindquist who is employee of Data Sciences International, St. Paul, MN, USA. The authors declare no conflict of interest in this study.
Ethical approval
The study protocol and experimental procedures for using the animals were approved by the IACUC of Crown Bioscience Inc., which includes members from outside of the company. The approval number is AN-1308-016-19.
Informed consent
For this type of study formal consent is not required.
Figures




References
-
- Chatzigeorgiou A, Halapas A, Kalafatakis K, Kamper E. The use of animal models in the study of diabetes mellitus. In Vivo. 2009;23(2):245–258. - PubMed
-
- Wang X, Wang B, Sun G, Wu J, Liu Y, Wang Y, Xiao YF. Dysglycemia and dyslipidemia models in nonhuman primates: part I. Model of naturally occurring diabetes. J Diabetes Metab. 2015;13:010.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

