Design of a pragmatic trial in minority children presenting to the emergency department with uncontrolled asthma: The CHICAGO Plan
- PMID: 28366780
- PMCID: PMC5496921
- DOI: 10.1016/j.cct.2017.03.015
Design of a pragmatic trial in minority children presenting to the emergency department with uncontrolled asthma: The CHICAGO Plan
Abstract
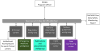
Among children with asthma, black children are two to four times as likely to have an emergency department (ED) visit and die from asthma, respectively, compared to white children in the United States. Despite the availability of evidence-based asthma management guidelines, minority children are less likely than white children to receive or use effective options for asthma care. The CHICAGO Plan is a three-arm multi-center randomized pragmatic trial of children 5 to 11years old presenting to the ED with uncontrolled asthma that compares: [1] an ED-focused intervention to improve the quality of care on discharge to home, [2] the same ED-focused intervention together with a home-based community health worker (CHW)-led intervention, and [3] enhanced usual care. All children receive spacers for the metered dose inhaler and teaching about its use. The Patient-Reported Outcomes Measurement Information System (PROMIS) Asthma Impact Scale and Satisfaction with Participation in Social Roles at 6months are the primary outcomes in children and in caregivers, respectively. Other patient-reported outcomes and indicators of healthcare utilization are assessed as secondary outcomes. Innovative features of the CHICAGO Plan include early and continuous engagement of children, caregivers, the Chicago Department of Public Health, and other stakeholders to inform the design and implementation of the study and a shared research infrastructure to coordinate study activities. The objective of this report is to describe the development of the CHICAGO Plan, including the methods and rationale for engaging stakeholders, the shared research infrastructure, and other features of the pragmatic clinical trial design.
Trial registration: ClinicalTrials.gov NCT02319967.
Keywords: Community health worker; Health disparities; Pragmatic clinical trial; Quality of asthma care; Stakeholder engagement; Uncontrolled asthma.
Published by Elsevier Inc.
Figures


References
-
- U.S. Department of Health and Human Services (HHS) Expert panel report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: HHS, National Heart, Lung and Blood Institute, National Institutes of Health; 2007. [accessed March 17, 2017]. Publication No. 07–4051. http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines.
-
- Centers for Disease Control and Prevention. [accessed March 17, 2017];AsthmaStats. http://www.cdc.gov/asthma/asthma_stats/
-
- Moorman JE, Akinbami LJ, Bailey CM, et al. National Surveillance of Asthma: United States, 2001–2010. National Center for Health Statistics. Vital Health Stat. 2012;3(35) - PubMed
-
- Illinois Department of Public Health. [accessed March 17, 2017];Asthma burden update. 2013 http://www.iedasp.org/iedasp/AsthmaBurdenBrief_201305_final.pdf.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

