Proteomic and functional analyses reveal pleiotropic action of the anti-tumoral compound NBDHEX in Giardia duodenalis
- PMID: 28366863
- PMCID: PMC5377010
- DOI: 10.1016/j.ijpddr.2017.03.006
Proteomic and functional analyses reveal pleiotropic action of the anti-tumoral compound NBDHEX in Giardia duodenalis
Abstract
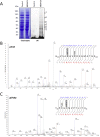
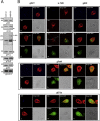
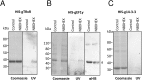
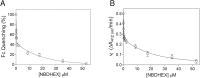
Giardiasis, a parasitic diarrheal disease caused by Giardia duodenalis, affects one billion people worldwide. Treatment relies only on a restricted armamentarium of drugs. The disease burden and the increase in treatment failure highlight the need for novel, safe and well characterized drug options. The antitumoral compound NBDHEX is effective in vitro against Giardia trophozoites and inhibits glycerol-3-phosphate dehydrogenase. Aim of this work was to search for additional NBDHEX protein targets. The intrinsic NBDHEX fluorescence was exploited in a proteomic analysis to select and detect modified proteins in drug treated Giardia. In silico structural analysis, intracellular localization and functional assays were further performed to evaluate drug effects on the identified targets. A small subset of Giardia proteins was covalently bound to the drug at specific cysteine residues. These proteins include metabolic enzymes, e.g. thioredoxin reductase (gTrxR), as well as elongation factor 1B-γ (gEF1Bγ), and structural proteins, e.g. α-tubulin. We showed that NBDHEX in vitro binds to recombinant gEF1Bγ and gTrxR, but only the last one could nitroreduce NBDHEX leading to drug modification of gTrxR catalytic cysteines, with concomitant disulphide reductase activity inhibition and NADPH oxidase activity upsurge. Our results indicate that NBDHEX reacts with multiple targets whose roles and/or functions are specifically hampered. In addition, NBDHEX is in turn converted to reactive intermediates extending its toxicity. The described NBDHEX pleiotropic action accounts for its antigiardial activity and encourages the use of this drug as a promising alternative for the future treatment of giardiasis.
Keywords: Elongation factor 1Bγ; Giardia; NBDHEX; Thioredoxin reductase.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Similar articles
-
Structural characterization of Giardia duodenalis thioredoxin reductase (gTrxR) and computational analysis of its interaction with NBDHEX.Eur J Med Chem. 2017 Jul 28;135:479-490. doi: 10.1016/j.ejmech.2017.04.057. Epub 2017 Apr 24. Eur J Med Chem. 2017. PMID: 28477573
-
The FAD-dependent glycerol-3-phosphate dehydrogenase of Giardia duodenalis: an unconventional enzyme that interacts with the g14-3-3 and it is a target of the antitumoral compound NBDHEX.Front Microbiol. 2015 Jun 1;6:544. doi: 10.3389/fmicb.2015.00544. eCollection 2015. Front Microbiol. 2015. PMID: 26082764 Free PMC article.
-
Quantitative proteomics in Giardia duodenalis-Achievements and challenges.Mol Biochem Parasitol. 2016 Aug;208(2):96-112. doi: 10.1016/j.molbiopara.2016.07.002. Epub 2016 Jul 19. Mol Biochem Parasitol. 2016. PMID: 27449313
-
Drug resistance in Giardia duodenalis.Biotechnol Adv. 2015 Nov 1;33(6 Pt 1):888-901. doi: 10.1016/j.biotechadv.2015.04.009. Epub 2015 Apr 25. Biotechnol Adv. 2015. PMID: 25922317 Review.
-
Proteomic analysis of Giardia: studies from the pre- and post-genomic era.Exp Parasitol. 2010 Jan;124(1):26-30. doi: 10.1016/j.exppara.2009.03.012. Epub 2009 Mar 24. Exp Parasitol. 2010. PMID: 19324039 Review.
Cited by
-
Click chemistry-facilitated comprehensive identification of proteins adducted by antimicrobial 5-nitroimidazoles for discovery of alternative drug targets against giardiasis.PLoS Negl Trop Dis. 2020 Apr 17;14(4):e0008224. doi: 10.1371/journal.pntd.0008224. eCollection 2020 Apr. PLoS Negl Trop Dis. 2020. PMID: 32302296 Free PMC article.
-
Re-Discovery of Giardiavirus: Genomic and Functional Analysis of Viruses from Giardia duodenalis Isolates.Biomedicines. 2021 Jun 8;9(6):654. doi: 10.3390/biomedicines9060654. Biomedicines. 2021. PMID: 34201207 Free PMC article.
-
Physiological aspects of nitro drug resistance in Giardia lamblia.Int J Parasitol Drugs Drug Resist. 2018 Aug;8(2):271-277. doi: 10.1016/j.ijpddr.2018.04.008. Epub 2018 Apr 28. Int J Parasitol Drugs Drug Resist. 2018. PMID: 29738984 Free PMC article.
-
Transmission-Blocking Strategies for Malaria Eradication: Recent Advances in Small-Molecule Drug Development.Pharmaceuticals (Basel). 2024 Jul 19;17(7):962. doi: 10.3390/ph17070962. Pharmaceuticals (Basel). 2024. PMID: 39065810 Free PMC article. Review.
-
The Nitrobenzoxadiazole Derivative NBDHEX Behaves as Plasmodium falciparum Gametocyte Selective Inhibitor with Malaria Parasite Transmission Blocking Activity.Pharmaceuticals (Basel). 2022 Jan 29;15(2):168. doi: 10.3390/ph15020168. Pharmaceuticals (Basel). 2022. PMID: 35215282 Free PMC article.
References
-
- Achilonu I., Siganunu T.P., Dirr H.W. Purification and characterisation of recombinant human eukaryotic elongation factor 1 gamma. Protein Expr. Purif. 2014;99:70–77. - PubMed
-
- Aguayo-Ortiz R., Méndez-Lucio O., Romo-Mancillas A., Castillo R., Yépez-Mulia L., Medina-Franco J.L., Hernández-Campos A. Molecular basis for benzimidazole resistance from a novel β-tubulin binding site model. J. Mol. Graph. Model. 2013;45:26–37. - PubMed
-
- Ansell B.R., McConville M.J., Ma'ayeh S.Y., Dagley M.J., Gasser R.B., Svärd S.G., Jex A.R. Drug resistance in Giardia duodenalis. Biotechnol. Adv. 2015;33:888–901. - PubMed
-
- Arias D.G., Regner E.L., Iglesias A.A., Guerrero S.A. Entamoeba histolytica thioredoxin reductase: molecular and functional characterization of its atypical properties. Biochim. Biophys. Acta. 2012;1820:1859–1866. - PubMed
-
- Bagchi S., Oniku A.E., Topping K., Mamhoud Z.N., Paget T.A. Programmed cell death in Giardia. Parasitology. 2012;139:894–903. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

