A novel AHI-1-BCR-ABL-DNM2 complex regulates leukemic properties of primitive CML cells through enhanced cellular endocytosis and ROS-mediated autophagy
- PMID: 28366933
- PMCID: PMC5668499
- DOI: 10.1038/leu.2017.108
A novel AHI-1-BCR-ABL-DNM2 complex regulates leukemic properties of primitive CML cells through enhanced cellular endocytosis and ROS-mediated autophagy
Abstract
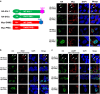
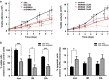
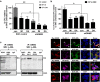
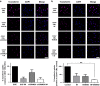
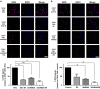
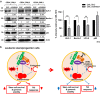
Tyrosine kinase inhibitor (TKI) therapies induce clinical remission with remarkable effects on chronic myeloid leukemia (CML). However, very few TKIs completely eradicate the leukemic clone and persistence of leukemic stem cells (LSCs) remains challenging, warranting new, distinct targets for improved treatments. We demonstrated that the scaffold protein AHI-1 is highly deregulated in LSCs and interacts with multiple proteins, including Dynamin-2 (DNM2), to mediate TKI-resistance of LSCs. We have now demonstrated that the SH3 domain of AHI-1 and the proline rich domain of DNM2 are mainly responsible for this interaction. DNM2 expression was significantly increased in CML stem/progenitor cells; knockdown of DNM2 greatly impaired their survival and sensitized them to TKI treatments. Importantly, a new AHI-1-BCR-ABL-DNM2 protein complex was uncovered, which regulates leukemic properties of these cells through a unique mechanism of cellular endocytosis and ROS-mediated autophagy. Thus, targeting this complex may facilitate eradication of LSCs for curative therapies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Molecular and structural characterization of the SH3 domain of AHI-1 in regulation of cellular resistance of BCR-ABL(+) chronic myeloid leukemia cells to tyrosine kinase inhibitors.Proteomics. 2012 Jul;12(13):2094-106. doi: 10.1002/pmic.201100553. Proteomics. 2012. PMID: 22623184
-
AHI-1 interacts with BCR-ABL and modulates BCR-ABL transforming activity and imatinib response of CML stem/progenitor cells.J Exp Med. 2008 Oct 27;205(11):2657-71. doi: 10.1084/jem.20072316. Epub 2008 Oct 20. J Exp Med. 2008. PMID: 18936234 Free PMC article.
-
Targeting BCR-ABL+ stem/progenitor cells and BCR-ABL-T315I mutant cells by effective inhibition of the BCR-ABL-Tyr177-GRB2 complex.Oncotarget. 2017 Jul 4;8(27):43662-43677. doi: 10.18632/oncotarget.18216. Oncotarget. 2017. PMID: 28599273 Free PMC article.
-
Reactive oxygen species in BCR-ABL1-expressing cells - relevance to chronic myeloid leukemia.Acta Biochim Pol. 2017;64(1):1-10. doi: 10.18388/abp.2016_1396. Epub 2016 Dec 1. Acta Biochim Pol. 2017. PMID: 27904889 Review.
-
Molecular pathogenesis of chronic myeloid leukemia: implications for new therapeutic strategies.Ann Hematol. 1999 Feb;78(2):49-64. doi: 10.1007/s002770050473. Ann Hematol. 1999. PMID: 10089019 Review.
Cited by
-
Vitamin C Inhibits Lipopolysaccharide-Induced Hyperinflammatory State of Chronic Myeloid Leukemia Cells through Purinergic Signaling and Autophagy.Nutrients. 2024 Jan 29;16(3):383. doi: 10.3390/nu16030383. Nutrients. 2024. PMID: 38337668 Free PMC article.
-
Properties of Leukemic Stem Cells in Regulating Drug Resistance in Acute and Chronic Myeloid Leukemias.Biomedicines. 2022 Jul 30;10(8):1841. doi: 10.3390/biomedicines10081841. Biomedicines. 2022. PMID: 36009388 Free PMC article. Review.
-
A Joint Model of Random Forest and Artificial Neural Network for the Diagnosis of Endometriosis.Front Genet. 2022 Mar 8;13:848116. doi: 10.3389/fgene.2022.848116. eCollection 2022. Front Genet. 2022. PMID: 35350240 Free PMC article.
-
Autophagy and Metabolism in Normal and Malignant Hematopoiesis.Int J Mol Sci. 2021 Aug 9;22(16):8540. doi: 10.3390/ijms22168540. Int J Mol Sci. 2021. PMID: 34445246 Free PMC article. Review.
-
Phosphorylation of BECLIN-1 by BCR-ABL suppresses autophagy in chronic myeloid leukemia.Haematologica. 2020 May;105(5):1285-1293. doi: 10.3324/haematol.2018.212027. Epub 2019 Aug 8. Haematologica. 2020. PMID: 31399521 Free PMC article.
References
-
- Druker BJ, O'Brien SG, Cortes J, Radich J. Chronic myelogenous leukemia. Hematol Am Soc Hematol Educ Program 2002, 111–135. - PubMed
-
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med 1999; 340: 1330–1340. - PubMed
-
- Savona M, Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nat Rev Cancer 2008; 8: 341–350. - PubMed
-
- Jiang X, Smith C, Eaves A, Eaves C. The challenges of targeting chronic myeloid leukemia stem cells. Clin Lymphoma Myeloma 2007; 7 (Suppl 2): S71–S80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

