Novel approaches to vitiligo treatment via modulation of mTOR and NF-κB pathways in human skin melanocytes
- PMID: 28367103
- PMCID: PMC5370446
- DOI: 10.7150/ijbs.17318
Novel approaches to vitiligo treatment via modulation of mTOR and NF-κB pathways in human skin melanocytes
Abstract
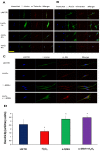
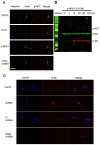
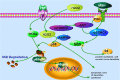
Vitiligo is a skin depigmentation disorder with an increasing prevalence. Among recognized mechanisms is the oxidative stress that affects melanocytes which are responsible for skin pigmentation. Studies have shown that high concentration of hydrogen peroxide, or H2O2, induces apoptotic activities. Few studies have been done with lower doses of H2O2. Using human skin melanocytes, we investigated the effect of moderate concentration of H2O2 on melanocyte dendrites. Confocal data show that H2O2 at 250 µM induces loss of dendrites, as indicated by cytoskeletal proteins. α-melanocyte stimulating hormone or α-MSH pretreatment protects against H2O2-induced loss of dendrites, while α-MSH alone enhances dendrites. PI3K/AKT inhibitor LY294002 and mTORC1 inhibitor Rapamycin inhibit α-MSH-induced dendrites. In this study, we also investigated the effect of TNFα on cultured human skin melanocytes, since TNFα plays important roles in vitiligo. Confocal data demonstrate that TNFα induces NFκB activation. Western blot analysis shows that TNFα induces IκB phosphorylation and degradation. Interestingly, α-MSH does not have any effect of TNFα-induced IκB degradation and NF-κB activation. α-MSH, however, activates mTORC1 pathway. TNFα induces p38 but not AMPKα activation. Collectively, our data suggest that modulation of mTOR and NF-κB pathways may be a novel approach for better clinical management of vitiligo.
Keywords: TNFα; mTORC1; vitiligo; α-MSH.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes.Mol Cancer Res. 2012 Jun;10(6):778-86. doi: 10.1158/1541-7786.MCR-11-0436. Epub 2012 May 23. Mol Cancer Res. 2012. PMID: 22622028
-
Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway.Free Radic Biol Med. 2018 Dec;129:492-503. doi: 10.1016/j.freeradbiomed.2018.10.421. Epub 2018 Oct 18. Free Radic Biol Med. 2018. PMID: 30342186
-
Ginsenoside Rk1 protects human melanocytes from H2O2‑induced oxidative injury via regulation of the PI3K/AKT/Nrf2/HO‑1 pathway.Mol Med Rep. 2021 Nov;24(5):821. doi: 10.3892/mmr.2021.12462. Epub 2021 Sep 24. Mol Med Rep. 2021. PMID: 34558653 Free PMC article.
-
The role of tumor necrosis factor-α in the pathogenesis of vitiligo.Am J Clin Dermatol. 2013 Oct;14(5):343-50. doi: 10.1007/s40257-013-0039-3. Am J Clin Dermatol. 2013. PMID: 23912226 Review.
-
Polyphenols targeting multiple molecular targets and pathways for the treatment of vitiligo.Front Immunol. 2024 Jul 25;15:1387329. doi: 10.3389/fimmu.2024.1387329. eCollection 2024. Front Immunol. 2024. PMID: 39119340 Free PMC article. Review.
Cited by
-
Differentially expressed microRNAs in peripheral blood mononuclear cells of non-segmental vitiligo and their clinical significance.J Clin Lab Anal. 2021 Feb;35(2):e23648. doi: 10.1002/jcla.23648. Epub 2020 Nov 10. J Clin Lab Anal. 2021. PMID: 33169883 Free PMC article.
-
Unraveling genetic predisposition and oxidative stress in vitiligo development and the role of artificial intelligence (AI) in diagnosis and management.J Med Biochem. 2025 Jul 4;44(4):713-723. doi: 10.5937/jomb0-56661. J Med Biochem. 2025. PMID: 40837363 Free PMC article.
-
A Systematic Study of Yiqi Qubai Standard Decoction for Treating Vitiligo Based on UPLC-Q-TOF/MS Combined with Chemometrics, Molecular Docking, and Cellular and Zebrafish Assays.Pharmaceuticals (Basel). 2023 Dec 11;16(12):1716. doi: 10.3390/ph16121716. Pharmaceuticals (Basel). 2023. PMID: 38139842 Free PMC article.
-
The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders.Cells. 2021 May 17;10(5):1219. doi: 10.3390/cells10051219. Cells. 2021. PMID: 34067630 Free PMC article. Review.
-
Bioinformatics meets machine learning: identifying circulating biomarkers for vitiligo across blood and tissues.Front Immunol. 2025 May 15;16:1543355. doi: 10.3389/fimmu.2025.1543355. eCollection 2025. Front Immunol. 2025. PMID: 40443672 Free PMC article.
References
-
- Kruger C, Schallreuter KU. Cumulative life course impairment in vitiligo. Curr Probl Dermatol. 2013;44:102–17. - PubMed
-
- Kruger C, Panske A, Schallreuter KU. Disease-related behavioral patterns and experiences affect quality of life in children and adolescents with vitiligo. Int J Dermatol; 2013. - PubMed
-
- Malhotra N, Dytoc M. The pathogenesis of vitiligo. J Cutan Med Surg. 2013;17:153–72. - PubMed
-
- Alikhan A, Griffin J, Nguyen N, Davis DM, Gibson LE. Pediatric follicular mucinosis: presentation, histopathology, molecular genetics, treatment, and outcomes over an 11-year period at the Mayo Clinic. Pediatr Dermatol. 2013;30:192–8. - PubMed
-
- Le Poole C, Boissy RE. Vitiligo. Semin Cutan Med Surg. 1997;16:3–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

