Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications
- PMID: 28367220
- PMCID: PMC5359455
- DOI: 10.1155/2017/1594074
Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications
Abstract
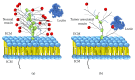
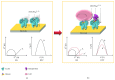
Lectins are proteins extensively used in biomedical applications with property to recognize carbohydrates through carbohydrate-binding sites, which identify glycans attached to cell surfaces, glycoconjugates, or free sugars, detecting abnormal cells and biomarkers related to diseases. These lectin abilities promoted interesting results in experimental treatments of immunological diseases, wounds, and cancer. Lectins obtained from virus, microorganisms, algae, animals, and plants were reported as modulators and tool markers in vivo and in vitro; these molecules also play a role in the induction of mitosis and immune responses, contributing for resolution of infections and inflammations. Lectins revealed healing effect through induction of reepithelialization and cicatrization of wounds. Some lectins have been efficient agents against virus, fungi, bacteria, and helminths at low concentrations. Lectin-mediated bioadhesion has been an interesting characteristic for development of drug delivery systems. Lectin histochemistry and lectin-based biosensors are useful to detect transformed tissues and biomarkers related to disease occurrence; antitumor lectins reported are promising for cancer therapy. Here, we address lectins from distinct sources with some biological effect and biotechnological potential in the diagnosis and therapeutic of diseases, highlighting many advances in this growing field.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

