Upgrading syngas fermentation effluent using Clostridium kluyveri in a continuous fermentation
- PMID: 28367228
- PMCID: PMC5372331
- DOI: 10.1186/s13068-017-0764-6
Upgrading syngas fermentation effluent using Clostridium kluyveri in a continuous fermentation
Abstract
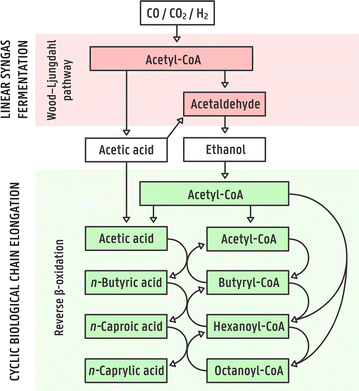
Background: The product of current syngas fermentation systems is an ethanol/acetic acid mixture and the goal is to maximize ethanol recovery. However, ethanol currently has a relatively low market value and its separation from the fermentation broth is energy intensive. We can circumvent these disadvantages of ethanol production by converting the dilute ethanol/acetic acid mixture into products with longer carbon backbones, which are of higher value and are more easily extracted than ethanol. Chain elongation, which is the bioprocess in which ethanol is used to elongate short-chain carboxylic acids to medium-chain carboxylic acids (MCCAs), has been studied with pure cultures and open cultures of microbial consortia (microbiomes) with several different substrates. While upgrading syngas fermentation effluent has been studied with open cultures, to our knowledge, no study exists that has performed this with pure cultures.
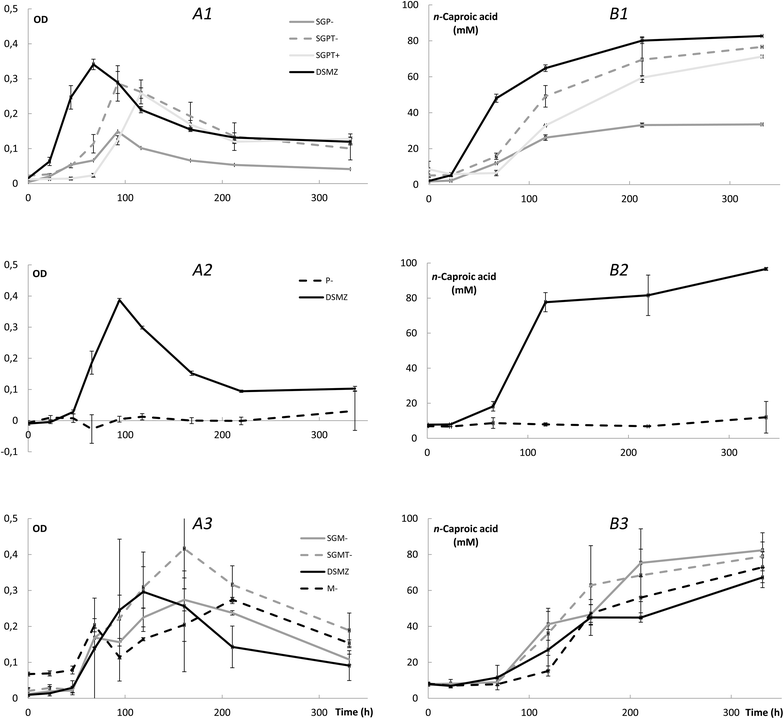
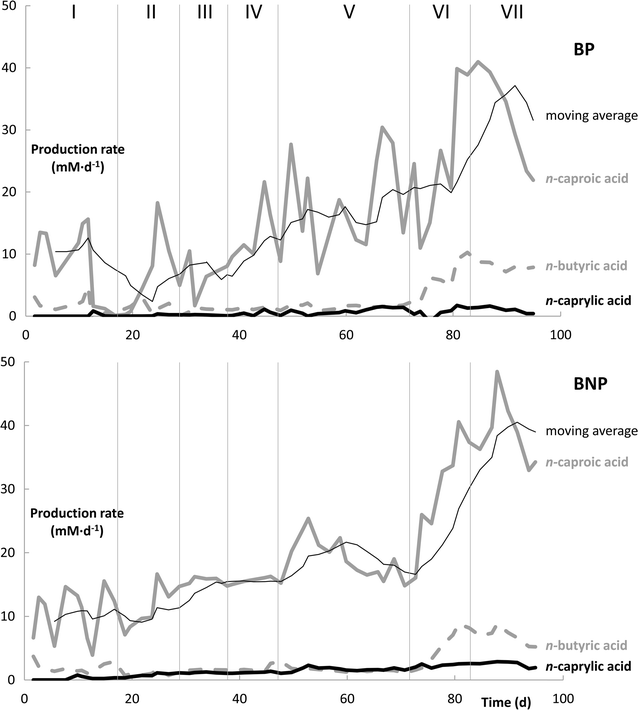
Results: Here, pure cultures of Clostridium kluyveri were used in continuous bioreactors to convert ethanol/acetic acid mixtures into MCCAs. Besides changing the operating conditions in regards to substrate loading rates and composition, the effect of in-line product extraction, pH, and the use of real syngas fermentation effluent on production rates were tested. Increasing the organic loading rates resulted in proportionally higher production rates of n-caproic acid, which were up to 40 mM day-1 (4.64 g L-1 day-1) at carbon conversion efficiencies of 90% or higher. The production rates were similar for bioreactors with and without in-line product extraction. Furthermore, a lower ethanol/acetic acid ratio (3:1 instead of 10:1) enabled faster and more efficient n-caproic acid production. In addition, n-caprylic acid production was observed for the first time with C. kluyveri (up to 2.19 ± 0.34 mM in batch). Finally, the use of real effluent from syngas fermentation, without added yeast extract, but with added defined growth factors, did maintain similar production rates. Throughout the operating period, we observed that the metabolism of C. kluyveri was inhibited at a mildly acidic pH value of 5.5 compared to a pH value of 7.0, while reactor microbiomes perform successfully at mildly acidic conditions.
Conclusions: Clostridium kluyveri can be used as a biocatalyst to upgrade syngas fermentation effluent into MCCAs at pH values above 5.5.
Keywords: Carboxylate platform; Carboxylic acids; Chain elongation; Syngas fermentation; n-Caproic acid; n-Caprylic acid.
Figures

Similar articles
-
Upgrading dilute ethanol from syngas fermentation to n-caproate with reactor microbiomes.Bioresour Technol. 2014 Jan;151:378-82. doi: 10.1016/j.biortech.2013.09.105. Epub 2013 Oct 3. Bioresour Technol. 2014. PMID: 24140415
-
Integrating syngas fermentation with the carboxylate platform and yeast fermentation to reduce medium cost and improve biofuel productivity.Environ Technol. 2013 Jul-Aug;34(13-16):1983-94. doi: 10.1080/09593330.2013.826255. Environ Technol. 2013. PMID: 24350452
-
Biochar and activated carbon enhance ethanol conversion and selectivity to caproic acid by Clostridium kluyveri.Bioresour Technol. 2021 Jan;319:124236. doi: 10.1016/j.biortech.2020.124236. Epub 2020 Oct 8. Bioresour Technol. 2021. PMID: 33254460
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
-
Enhanced solventogenesis in syngas bioconversion: Role of process parameters and thermodynamics.Chemosphere. 2022 Jul;299:134425. doi: 10.1016/j.chemosphere.2022.134425. Epub 2022 Mar 26. Chemosphere. 2022. PMID: 35351479 Review.
Cited by
-
Synthetic co-culture of autotrophic Clostridium carboxidivorans and chain elongating Clostridium kluyveri monitored by flow cytometry.Microb Biotechnol. 2022 May;15(5):1471-1485. doi: 10.1111/1751-7915.13941. Epub 2021 Oct 20. Microb Biotechnol. 2022. PMID: 34669248 Free PMC article.
-
Product Inhibition and pH Affect Stoichiometry and Kinetics of Chain Elongating Microbial Communities in Sequencing Batch Bioreactors.Front Bioeng Biotechnol. 2021 Jun 21;9:693030. doi: 10.3389/fbioe.2021.693030. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34235138 Free PMC article.
-
Functional genome-centric view of the CO-driven anaerobic microbiome.ISME J. 2021 Oct;15(10):2906-2919. doi: 10.1038/s41396-021-00983-1. Epub 2021 Apr 28. ISME J. 2021. PMID: 33911204 Free PMC article.
-
Implementation of a Clostridium luticellarii genome-scale model for upgrading syngas fermentations.Comput Struct Biotechnol J. 2025 Jan 22;27:649-660. doi: 10.1016/j.csbj.2025.01.013. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40046987 Free PMC article.
-
Caproate production from Enset fiber in one-pot two-step fermentation using anaerobic fungi (Neocallimastix cameroonii strain G341) and Clostridium kluyveri DSM 555.Microb Cell Fact. 2023 Oct 20;22(1):216. doi: 10.1186/s12934-023-02224-w. Microb Cell Fact. 2023. PMID: 37864174 Free PMC article.
References
-
- Opportunities for the fermentation-based chemical industry: an analysis of the market potential and competitiveness of North-West Europe [http://www2.deloitte.com/global/en/pages/manufacturing/articles/opportun...]. Accessed 22 Mar 2017.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

