Efficacy of Cabazitaxel Treatment in Metastatic Castration Resistant Prostate Cancer in Second and Later Lines. An Experience from Two German Centers
- PMID: 28367230
- PMCID: PMC5370494
- DOI: 10.7150/jca.17644
Efficacy of Cabazitaxel Treatment in Metastatic Castration Resistant Prostate Cancer in Second and Later Lines. An Experience from Two German Centers
Abstract
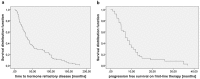
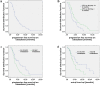
Purpose: Several new treatment options for patients with metastatic castration resistant prostate cancer (mCRPC) have been approved within the last years - among them cabazitaxel (CAB), abiraterone acetate, enzalutamide, and radium-223. The aim of this study was to assess factors predictive for efficacy of CAB. Methods: We analyzed all patients with mCRPC treated with CAB at our institutions between 2011 and 2016. Data were retrieved retrospectively from the electronical patient chart. Results: 69 patients received CAB (26.1% 2nd line, 36.2% 3rd line, 37.3% >3rd line). Median overall survival (OS) on CAB was 10.0 months (95%CI 7.1-12.9). Median progression free survival (PFS) on CAB was 3.9 months (95%CI 3.0-4.8). There were no differences in OS and PFS regarding treatment line of CAB (2nd vs. higher; 2nd/3rd vs. higher). Duration of remission on 1st line treatment (> 6 months vs. </= 6 months) was associated with a longer PFS with subsequent CAB treatment (4.1 months vs. 3.0 months (95%CI 3.0-5.2; 2.2-3.8); p=0.021). Patients with visceral metastases had a shorter PFS (3.0 months; 95%CI 2.6-3.3) and OS (8.7 months; 95%CI 5.9-11.5) on CAB compared to patients who had bone and/or lymph node lesions only (PFS: 5.8 months; 95%CI 3.2-8.4; p=0.014; OS: 11.7 months; 95%CI 7.5-15.9; p=0.042). Conclusions: Results from our patient cohort suggest that a longer PFS to any 1st line treatment for mCRPC is correlated with a longer PFS to CAB for any later line treatment. Patients with nodal and bone metastases only had a significantly superior PFS and OS with CAB treatment than patients with visceral metastases.
Keywords: Cabazitaxel; castration resistance.; prostate cancer; sequencing therapy; survival.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Tannock IF, de Wit R, Berry WR. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- de Bono JS, Oudard S, Ozguroglu M. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. - PubMed
-
- Fizazi K, Scher HI, Molina A. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. - PubMed
-
- Scher HI, Fizazi K, Saad F. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

