Spermidine Oxidation-Mediated Degeneration of Retinal Pigment Epithelium in Rats
- PMID: 28367269
- PMCID: PMC5359532
- DOI: 10.1155/2017/4128061
Spermidine Oxidation-Mediated Degeneration of Retinal Pigment Epithelium in Rats
Abstract
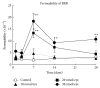
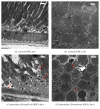
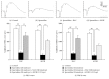
Retinal pigment epithelium (RPE) degeneration is a crucial event in dry age-related macular degeneration and gyrate atrophy. The polyamine spermidine has been shown to induce RPE cell death in vitro. The present study aimed to establish a novel in vivo model of spermidine-induced RPE degeneration and to determine whether spermidine-induced RPE cell death involves oxidative mechanisms. In this study, spermidine caused ARPE-19 cell death in a concentration-dependent manner. This effect was prevented by removal of serum from the culture medium or treatment with amine oxidase inhibitors, N-acetylcysteine (NAC), or aldehyde dehydrogenase (ALDH). Intravitreal injection of spermidine into rats significantly increased the permeability of the blood-retinal barrier and decreased the amplitudes of scotopic electroretinogram a- and b-waves. Histological analysis revealed that spermidine induced vacuolation, atrophy, and dropout of RPE cells, leading to the disruption of photoreceptor outer segments. Simultaneous intravitreal administration of NAC and ALDH with spermidine prominently inhibited the functional and morphological changes induced by spermidine. In conclusion, this study demonstrated that the intravitreal administration of spermidine induced RPE cell dysfunction and death followed by photoreceptor degeneration in rats. These effects of spermidine are thought to be mediated by oxidative stress and a toxic aldehyde generated during spermidine oxidation.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

