Transcriptome analysis of microglia in a mouse model of Rett syndrome: differential expression of genes associated with microglia/macrophage activation and cellular stress
- PMID: 28367307
- PMCID: PMC5372344
- DOI: 10.1186/s13229-017-0134-z
Transcriptome analysis of microglia in a mouse model of Rett syndrome: differential expression of genes associated with microglia/macrophage activation and cellular stress
Abstract
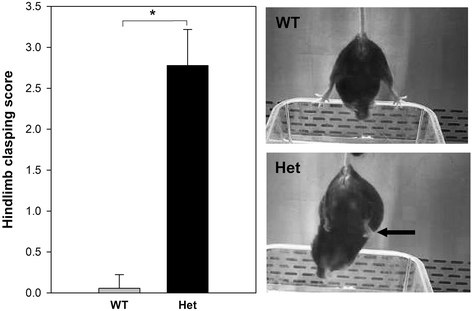
Background: Rett syndrome (RTT) is a severe, neurodevelopmental disorder primarily affecting girls, characterized by progressive loss of cognitive, social, and motor skills after a relatively brief period of typical development. It is usually due to de novo loss of function mutations in the X-linked gene, MeCP2, which codes for the gene expression and chromatin regulator, methyl-CpG binding protein 2. Although the behavioral phenotype appears to be primarily due to neuronal Mecp2 deficiency in mice, other cell types, including astrocytes and oligodendrocytes, also appear to contribute to some aspects of the RTT phenotype. In addition, microglia may also play a role. However, the effect of Mecp2 deficiency in microglia on RTT pathogenesis is controversial.
Methods: In the current study, we applied whole transcriptome analysis using RNA-seq to gain insight into molecular pathways in microglia that might be dysregulated during the transition, in female mice heterozygous for an Mecp2-null allele (Mecp2+/-; Het), from the pre-phenotypic (5 weeks) to the phenotypic phases (24 weeks).
Results: We found a significant overlap in differentially expressed genes (DEGs) with genes involved in regulating the extracellular matrix, and those that are activated or inhibited when macrophages and microglia are stimulated towards the M1 and M2 activation states. However, the M1- and M2-associated genes were different in the 5- and 24-week samples. In addition, a substantial decrease in the expression of nine members of the heat shock protein (HSP) family was found in the 5-week samples, but not at 24 weeks.
Conclusions: These findings suggest that microglia from pre-phenotypic and phenotypic female mice are activated in a manner different from controls and that pre-phenotypic female mice may have alterations in their capacity to response to heat stress and other stressors that function through the HSP pathway.
Keywords: Autism; Heat shock; Innate immune system; M1 activation; M2 activation; Microglia; Rett syndrome; Schizophrenia.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

