Mild, Redox-Neutral Alkylation of Imines Enabled by an Organic Photocatalyst
- PMID: 28367354
- PMCID: PMC5369175
- DOI: 10.1021/acscatal.6b03665
Mild, Redox-Neutral Alkylation of Imines Enabled by an Organic Photocatalyst
Abstract
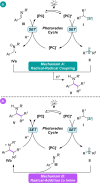
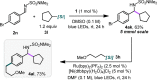
An operationally simple, mild, redox-neutral method for the photoredox alkylation of imines is reported. Utilizing an inexpensive organic photoredox catalyst, alkyl radicals are readily generated from the single-electron oxidation of ammonium alkyl bis(catecholato)silicates and are subsequently engaged in a C-C bond-forming reaction with imines. The process is highly selective, metal-free, and does not require a large excess of the alkylating reagent or the use of acidic additives.
Keywords: hypervalent silicon; imines; photocatalysis; radical alkylation; visible light.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
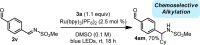
Similar articles
-
Silicates as Latent Alkyl Radical Precursors: Visible-Light Photocatalytic Oxidation of Hypervalent Bis-Catecholato Silicon Compounds.Angew Chem Int Ed Engl. 2015 Sep 21;54(39):11414-8. doi: 10.1002/anie.201504963. Epub 2015 Jul 24. Angew Chem Int Ed Engl. 2015. PMID: 26216069
-
Photocatalytic Alkyl Addition to Access Quaternary Alkynyl α-Amino Esters.Org Lett. 2022 Dec 9;24(48):8870-8874. doi: 10.1021/acs.orglett.2c03669. Epub 2022 Nov 22. Org Lett. 2022. PMID: 36414400
-
Cross-Coupling of Alkyl Redox-Active Esters with Benzophenone Imines: Tandem Photoredox and Copper Catalysis.Angew Chem Int Ed Engl. 2018 Jul 20;57(30):9501-9504. doi: 10.1002/anie.201804873. Epub 2018 Jun 28. Angew Chem Int Ed Engl. 2018. PMID: 29863760
-
Radical carbon-carbon bond formations enabled by visible light active photocatalysts.Chimia (Aarau). 2012;66(6):394-8. doi: 10.2533/chimia.2012.394. Chimia (Aarau). 2012. PMID: 22871282 Review.
-
Light-Mediated Radical Addition to Azomethine Compounds: Novel Reactivity and Activation Modes.Chem Rec. 2025 Jan;25(1):e202400194. doi: 10.1002/tcr.202400194. Epub 2024 Dec 17. Chem Rec. 2025. PMID: 39690857 Review.
Cited by
-
Photoredox Generation of Carbon-Centered Radicals Enables the Construction of 1,1-Difluoroalkene Carbonyl Mimics.Angew Chem Int Ed Engl. 2017 Nov 20;56(47):15073-15077. doi: 10.1002/anie.201709487. Epub 2017 Oct 24. Angew Chem Int Ed Engl. 2017. PMID: 28960656 Free PMC article.
-
Photoredox-Catalyzed Oxidation of Anions for the Atom-Economical Hydro-, Amido-, and Dialkylation of Alkenes.J Org Chem. 2022 Mar 4;87(5):3498-3510. doi: 10.1021/acs.joc.1c03055. Epub 2022 Feb 8. J Org Chem. 2022. PMID: 35133155 Free PMC article.
-
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis.Beilstein J Org Chem. 2020 May 29;16:1163-1187. doi: 10.3762/bjoc.16.103. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32550931 Free PMC article. Review.
-
Stereoselective synthesis of unnatural α-amino acid derivatives through photoredox catalysis.Chem Sci. 2021 Mar 3;12(15):5430-5437. doi: 10.1039/d1sc00658d. Chem Sci. 2021. PMID: 34168785 Free PMC article.
-
Allylic C(sp3)-H alkylation via synergistic organo- and photoredox catalyzed radical addition to imines.Chem Sci. 2020 Apr 30;11(19):4954-4959. doi: 10.1039/d0sc00819b. Chem Sci. 2020. PMID: 34122952 Free PMC article.
References
-
- Prier C. K.; Rankic D. A.; Macmillan D. W. C. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. - DOI - PMC - PubMed
- Narayanam J. M. R.; Stephenson C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. 10.1039/B913880N. - DOI - PubMed
- Romero N. A.; Nicewicz D. A. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. - DOI - PubMed
- Shaw M. H.; Twilton J.; Macmillan D. W. C. J. Org. Chem. 2016, 81, 6898–6926. 10.1021/acs.joc.6b01449. - DOI - PMC - PubMed
-
-
For seminal reports, see:
- Tellis J. C.; Primer D. N.; Molander G. A. Science 2014, 345, 433–436. 10.1126/science.1253647. - DOI - PMC - PubMed
- Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Science 2014, 345, 437–440. 10.1126/science.1255525. - DOI - PMC - PubMed
-
For reviews, see:
- Tellis J. C.; Kelly C. B.; Primer D. N.; Jouffroy M.; Patel N. R.; Molander G. A. Acc. Chem. Res. 2016, 49, 1429–1439. 10.1021/acs.accounts.6b00214. - DOI - PMC - PubMed
- Skubi K. L.; Blum T. R.; Yoon T. P. Chem. Rev. 2016, 116, 10035–10074. 10.1021/acs.chemrev.6b00018. - DOI - PMC - PubMed
- Gui Y.-Y.; Sun L.; Lu Z.-P.; Yu D.-G. Org. Chem. Front. 2016, 3, 522–526. 10.1039/C5QO00437C. - DOI
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
