Increased long distance event-related gamma band connectivity in Alzheimer's disease
- PMID: 28367402
- PMCID: PMC5361871
- DOI: 10.1016/j.nicl.2017.02.021
Increased long distance event-related gamma band connectivity in Alzheimer's disease
Abstract
Background: Brain oscillatory responses can be used for non-invasive analyses of cortico-cortical connectivity, local neuronal synchronization, and coherence of oscillations in many neuropsychiatric conditions including Alzheimer's disease (AD). In the present paper, we examine sensory-evoked and event-related gamma coherences elicited by visual stimuli in three sub-gamma bands in two sub-groups of patients with AD (i.e., acetylcholinesterase-inhibitor treated and untreated) and healthy controls.
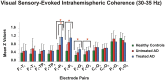
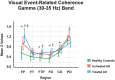
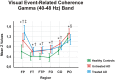
Methods: We studied a total of 39 patients with probable mild AD (according to NINCDS-ADRDA criteria) who had been sub-divided into untreated (n = 21) and treated (n = 18) (patients either on cholinergic monotherapy or combined therapy with memantine) AD groups, and 21 age-, gender-, and education-matched healthy elderly controls. A simple flash visual paradigm was applied for the acquisition of sensory-evoked coherences. Event-related coherences were elicited using a classical visual oddball paradigm. Both sensory-evoked and event-related gamma coherences were calculated for long-distance intrahemispheric pairs for three frequency ranges: 25-30 Hz, 30-35 Hz, and 40-48 Hz in post-stimulus 0-800 ms duration. The long-distance intrahemispheric pairs from both sides were fronto-parietal, fronto-temporal, fronto-temporoparietal, fronto-occipital, centro-occipital and parieto-occipital.
Results: The sensory-evoked or event-related gamma coherences revealed that both treated and untreated AD patients had significantly increased values compared to healthy controls in all three sub-gamma bands. Moreover, the treated AD patients demonstrated significantly higher fronto-parietal gamma coherences during both sensory stimulation and oddball paradigm and lower occipito-parietal coherences during oddball paradigm in comparison to untreated AD patients.
Conclusion: The present study demonstrated that an increase of gamma coherences was present in response to both visual sensory and cognitive stimulation in AD patients in all gamma sub-bands. Therefore, gamma oscillatory activity seems to be fundamental in brain functions at both the sensory and cognitive levels. The increase of gamma coherence values was not due to cholinergic treatment to any significant extent, as both treated and untreated AD patients had increased gamma coherence values compared to healthy controls. The use of coherence values reflecting brain connectivity holds potential for neuroimaging of AD and understanding brain dynamics related to the effects of medication.
Keywords: Alzheimer's disease; Coherence; Connectivity; EEG; Event-related; Gamma; Sensory-evoked.
Figures











References
-
- Babiloni C., Del Percio C., Boccardi M., Lizio R., Lopez S., Carducci F., Marzano N., Soricelli A., Ferri R., Triggiani A.I., Prestia A., Salinari S., Rasser P.E., Basar E., Famà F., Nobili F., Yener G., Emek-Savaş D.D., Gesualdo L., Mundi C., Thompson P.M., Rossini P.M., Frisoni G.B. Occipital sources of resting-state alpha rhythms are related to local gray matter density in subjects with amnesic mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2015;36(2):556–570. - PMC - PubMed
-
- Babiloni C., Triggiani A.I., Lizio R., Cordone S., Tattoli G., Bevilacqua V., Soricelli A., Ferri R., Nobili F., Gesualdo L., Millán-Calenti J.C., Buján A., Tortelli R., Cardinali V., Barulli M.R., Giannini A., Spagnolo P., Armenise S., Buenza G., Scianatico G., Logroscino G., Frisoni G.B., Del Percio C. Classification of single normal and Alzheimer's disease individuals from cortical sources of resting state EEG rhythms. Front. Neurosci. 2016;10:47. - PMC - PubMed
-
- Bajo R., Maestú F., Nevado A., Sancho M., Gutiérrez R., Campo P., Castellanos N.P., Gil P., Moratti S., Pereda E., Del-Pozo F. Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J. Alzheimers Dis. 2010;22(1):183–193. - PubMed
-
- Başar E. Elsevier; Amsterdam: 1980. EEG–Brain Dynamics. Relation Between EEG and Brain Evoked Potentials; pp. 1–411.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

