Synthetic Peptides as Potential Antigens for Cutaneous Leishmaniosis Diagnosis
- PMID: 28367456
- PMCID: PMC5359444
- DOI: 10.1155/2017/5871043
Synthetic Peptides as Potential Antigens for Cutaneous Leishmaniosis Diagnosis
Abstract
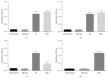
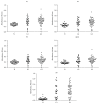
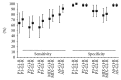
This work's goal was to research new candidate antigens for cutaneous leishmaniosis (CL). In order to reach the goal, we used random peptide phage display libraries screened using antibodies from Leishmania braziliensis patients. After selection, three peptides (P1, P2, and P3) were synthesized using Fmoc chemistry. The peptides individually or a mixture of them (MIX) was subsequently emulsified in complete and incomplete Freund's adjuvant and injected subcutaneously in golden hamsters. Sera from the hamsters administered with P1 presented antibodies that recognized proteins between 76 and 150 kDa from L. braziliensis. Sera from hamsters which had peptides P2 and P3, as well as the MIX, administered presented antibodies that recognized proteins between 52 and 76 kDa of L. braziliensis. The research on the similarity of the peptides' sequences in protein databases showed that they match a 63 kDa glycoprotein. The three peptides and the MIX were recognized by the sera from CL patients by immunoassay approach (ELISA). The peptides' MIX showed the best performance (79% sensitivity) followed by the P1 (72% sensitivity), and the AS presented 91% sensitivity. These results show a new route for discovering molecules for diagnosis or for immunoprotection against leishmaniosis.
Conflict of interest statement
The authors declare that they have no competing interests regarding the publication of this paper.
Figures

References
-
- Szargiki R., de Castro E. A., Luz E., Kowalthuk W., Machado Â. M., Thomaz-Soccol V. Comparison of serological and parasitological methods for cutaneous leishmaniasis diagnosis in the state of Paraná, Brazil. Brazilian Journal of Infectious Diseases. 2009;13(1):47–52. doi: 10.1590/S1413-86702009000100011. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

