Suppression of VEGF-induced angiogenesis and tumor growth by Eugenia jambolana, Musa paradisiaca, and Coccinia indica extracts
- PMID: 28367666
- PMCID: PMC6130448
- DOI: 10.1080/13880209.2017.1307422
Suppression of VEGF-induced angiogenesis and tumor growth by Eugenia jambolana, Musa paradisiaca, and Coccinia indica extracts
Abstract
Context: Abnormal angiogenesis and evasion of apoptosis are hallmarks of cancer. Accordingly, anti-angiogenic and pro-apoptotic therapies are effective strategies for cancer treatment. Medicinal plants, namely, Eugenia jambolana Lam. (Myrtaceae), Musa paradisiaca L. (Musaceae), and Coccinia indica Wight & Arn. (Cucurbitaceae), have not been greatly investigated for their anticancer potential.
Objective: We investigated the anti-angiogenic and pro-apoptotic efficacy of ethyl acetate (EA) and n-butanol (NB) extracts of E. jambolana (seeds), EA extracts of M. paradisiaca (roots) and C. indica (leaves) with respect to mammary neoplasia.
Materials and methods: Effect of extracts (2-200 μg/mL) on cytotoxicity and MCF-7, MDA-MB-231 and endothelial cell (EC) proliferation and in vitro angiogenesis were evaluated by MTT, 3[H]thymidine uptake and EC tube formation assays, respectively. In vivo tumour proliferation, VEGF secretion and angiogenesis were assessed using the Ehrlich ascites tumour (EAT) model followed by rat corneal micro-pocket and chicken chorioallantoic membrane (CAM) assays. Apoptosis induction was assessed by morphological and cell cycle analysis.
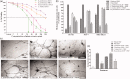
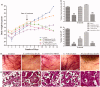
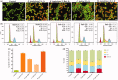
Results: EA extracts of E. jambolana and M. paradisiaca exhibited the highest cytotoxicity (IC50 25 and 60 μg/mL), inhibited cell proliferation (up to 81%), and tube formation (83% and 76%). In vivo treatment reduced body weight (50%); cell number (16.5- and 14.7-fold), secreted VEGF (∼90%), neoangiogenesis in rat cornea (2.5- and 1.5-fold) and CAM (3- and 1.6-fold) besides EAT cells accumulation in sub-G1 phase (20% and 18.38%), respectively.
Discussion and conclusion: Considering the potent anti-angiogenic and pro-apoptotic properties, lead molecules from EA extracts of E. jambolana and M. paradisiaca can be developed into anticancer drugs.
Keywords: Anti-angiogenic; cytotoxicity; pro-apoptotic.
Figures


References
-
- Ali EM, Sheta M, El Mohsen MA.. 2011. Elevated serum and tissue VEGF associated with poor outcome in breast cancer patients. Alexandria J Med. 47:217–224.
-
- Andzi-Barhé T, Massala KK, Obame LC, Engonga JL.. 2015. Phytochemical studies, total phenolic and flavonoids content and evaluation of antiradical activity of the extracts of the leaves from Dischistocalyx sp. (Acanthaceae). J Pharmacogn Phytochem. 3:174–178.
-
- Baliga MS.2011. Anticancer, chemopreventive and radioprotective potential of black plum (Eugenia jambolana Lam.). Asian Pac J Cancer Prev. 12:3–15. - PubMed
-
- Bikfalvi A, Bicknell R.. 2002. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends Pharmacol Sci. 23:576–582. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
