Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer's disease
- PMID: 28367951
- PMCID: PMC5382255
- DOI: 10.1038/ncomms14727
Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer's disease
Abstract
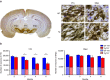
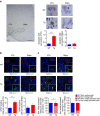
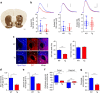
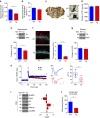
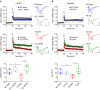
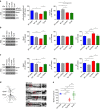
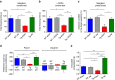
Alterations of the dopaminergic (DAergic) system are frequently reported in Alzheimer's disease (AD) patients and are commonly linked to cognitive and non-cognitive symptoms. However, the cause of DAergic system dysfunction in AD remains to be elucidated. We investigated alterations of the midbrain DAergic system in the Tg2576 mouse model of AD, overexpressing a mutated human amyloid precursor protein (APPswe). Here, we found an age-dependent DAergic neuron loss in the ventral tegmental area (VTA) at pre-plaque stages, although substantia nigra pars compacta (SNpc) DAergic neurons were intact. The selective VTA DAergic neuron degeneration results in lower DA outflow in the hippocampus and nucleus accumbens (NAc) shell. The progression of DAergic cell death correlates with impairments in CA1 synaptic plasticity, memory performance and food reward processing. We conclude that in this mouse model of AD, degeneration of VTA DAergic neurons at pre-plaque stages contributes to memory deficits and dysfunction of reward processing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Scheltens P. et al.. Alzheimer's disease. Lancet 388, 505–517 (2016). - PubMed
-
- D'Amelio M. & Rossini P. M. Brain excitability and connectivity of neuronal assemblies in Alzheimer's disease: from animal models to human findings. Prog. Neurobiol. 99, 42–60 (2012). - PubMed
-
- Scheff S. W., Price D. A., Schmitt F. A. & Mufson E. J. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging 27, 1372–1384 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

